Difference Between Sn1 And Sn2 Reaction
Juapaving
Apr 07, 2025 · 5 min read
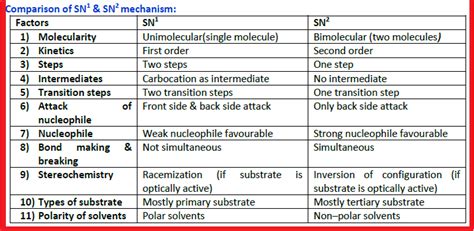
Table of Contents
Unveiling the Differences Between SN1 and SN2 Reactions: A Comprehensive Guide
Organic chemistry often presents a daunting landscape of reactions, mechanisms, and intricate details. Among these, the SN1 and SN2 reactions stand out as fundamental nucleophilic substitution reactions, yet they differ significantly in their mechanisms and characteristics. Understanding these differences is crucial for predicting reaction outcomes and designing efficient synthetic strategies. This comprehensive guide delves into the nuances of SN1 and SN2 reactions, highlighting their key distinctions and providing practical examples.
Understanding Nucleophilic Substitution Reactions
Before diving into the specifics of SN1 and SN2, let's establish a foundational understanding of nucleophilic substitution reactions. These reactions involve the replacement of a leaving group (typically a halide or a tosylate) on a carbon atom by a nucleophile, an electron-rich species seeking a positively charged center. The carbon atom bearing the leaving group is typically referred to as the electrophilic carbon. The overall process involves the breaking of a bond between the carbon and the leaving group and the formation of a new bond between the carbon and the nucleophile.
SN1 Reactions: A Step-by-Step Mechanism
SN1, or substitution nucleophilic unimolecular, reactions proceed through a two-step mechanism:
Step 1: Formation of a Carbocation
The first step involves the unimolecular departure of the leaving group, generating a carbocation intermediate. This step is the rate-determining step and is relatively slow. The stability of the carbocation directly influences the rate of the reaction. Tertiary carbocations are significantly more stable than secondary, and primary carbocations are the least stable. Therefore, SN1 reactions are favored by tertiary substrates.
Step 2: Nucleophilic Attack
The second step involves the attack of the nucleophile on the carbocation. This step is fast and doesn't influence the overall reaction rate. The nucleophile can attack the carbocation from either side, leading to a racemic mixture of products if the starting material is chiral.
Key Characteristics of SN1 Reactions:
- Rate = k[substrate] The rate only depends on the concentration of the substrate, hence "unimolecular."
- Favored by tertiary substrates: Tertiary carbocations are more stable.
- Weak nucleophiles are sufficient: The nucleophile participates in the faster second step.
- Protic solvents are preferred: Protic solvents stabilize the carbocation intermediate.
- Racemization: Leads to a racemic mixture due to planar carbocation intermediate.
- Rearrangements: Carbocation rearrangements can occur to form more stable carbocations.
SN2 Reactions: A Concerted Mechanism
SN2, or substitution nucleophilic bimolecular, reactions proceed through a concerted mechanism in a single step:
The Concerted Step: Backside Attack
In an SN2 reaction, the nucleophile attacks the electrophilic carbon from the backside, opposite to the leaving group. This backside attack causes inversion of configuration at the stereocenter if the substrate is chiral. The nucleophile, the substrate, and the leaving group are all involved in the transition state. This transition state is high in energy, making the reaction rate sensitive to steric hindrance.
Key Characteristics of SN2 Reactions:
- Rate = k[substrate][nucleophile] The rate depends on both the concentration of the substrate and the nucleophile, hence "bimolecular."
- Favored by primary substrates: Minimal steric hindrance allows for backside attack.
- Strong nucleophiles are required: The nucleophile actively participates in the rate-determining step.
- Aprotic solvents are preferred: Aprotic solvents do not hinder nucleophile's ability to attack.
- Inversion of configuration: Leads to inversion of stereochemistry at the stereocenter.
- No rearrangements: No intermediate is formed, thus no carbocation rearrangements are possible.
Head-to-Head Comparison: SN1 vs. SN2
| Feature | SN1 | SN2 |
|---|---|---|
| Mechanism | Two-step, carbocation intermediate | Concerted, one-step |
| Rate Law | Rate = k[substrate] | Rate = k[substrate][nucleophile] |
| Substrate | Tertiary > Secondary > Primary | Primary > Secondary > Tertiary (rare) |
| Nucleophile | Weak nucleophiles are sufficient | Strong nucleophiles are required |
| Solvent | Protic solvents preferred | Aprotic solvents preferred |
| Stereochemistry | Racemization (often) | Inversion of configuration |
| Rearrangements | Possible | Not possible |
| Leaving Group | Good leaving group is essential | Good leaving group is essential |
Factors Influencing SN1 and SN2 Reactions
Several factors beyond the substrate and nucleophile influence the preference for SN1 or SN2 pathways.
Leaving Group Ability:
A good leaving group is crucial for both SN1 and SN2 reactions. Good leaving groups are typically weak bases, meaning they are stable when they leave as anions. Examples include halides (I⁻ > Br⁻ > Cl⁻ > F⁻), tosylates, and mesylates.
Steric Hindrance:
Steric hindrance around the electrophilic carbon significantly impacts the reaction pathway. Bulky groups hinder the backside attack in SN2 reactions, favoring SN1. SN1 reactions are less sensitive to steric hindrance because the nucleophile attacks after the leaving group has departed.
Nucleophile Strength and Type:
Strong nucleophiles favor SN2 reactions, while weak nucleophiles are better suited for SN1 reactions. The nucleophile's size and charge also play a role; smaller, negatively charged nucleophiles are generally more reactive in SN2 reactions.
Solvent Effects:
Protic solvents, those capable of hydrogen bonding, stabilize the carbocation intermediate in SN1 reactions, increasing the reaction rate. Aprotic solvents, those lacking O-H or N-H bonds, are preferred for SN2 reactions as they do not solvate the nucleophile, leaving it more reactive.
Practical Applications and Examples
Understanding the differences between SN1 and SN2 reactions is crucial in many areas of organic chemistry. For example, in the synthesis of chiral molecules, a specific reaction pathway needs to be chosen to control stereochemistry.
Example 1: SN1 Reaction
The reaction of tert-butyl bromide with water in a protic solvent such as ethanol will proceed via an SN1 mechanism, producing tert-butyl alcohol as a racemic mixture.
Example 2: SN2 Reaction
The reaction of methyl bromide with sodium hydroxide in an aprotic solvent such as DMSO will proceed via an SN2 mechanism, producing methanol with inversion of configuration if the starting material was chiral.
Conclusion: Mastering the Nuances of SN1 and SN2
The SN1 and SN2 reactions represent fundamental concepts in organic chemistry, offering insights into reaction mechanisms and the factors influencing reaction pathways. By understanding the key distinctions between these reactions—their mechanisms, preferred substrates, nucleophiles, and solvents—chemists can predict reaction outcomes, design efficient syntheses, and control stereochemical outcomes. While this guide provides a comprehensive overview, further exploration of specific examples and advanced concepts will solidify your understanding of these crucial nucleophilic substitution pathways. Mastering these concepts lays a solid foundation for tackling more complex organic chemistry challenges.
Latest Posts
Latest Posts
-
Does An Earthworm Have A Backbone
Apr 10, 2025
-
What Is The Serous Membrane That Encloses Each Lung
Apr 10, 2025
-
Muscular Wall Separating The Abdominal And Thoracic Cavities
Apr 10, 2025
-
What Is A Net Force In Physics
Apr 10, 2025
-
Is The Five Carbon Sugar Found In Dna
Apr 10, 2025
Related Post
Thank you for visiting our website which covers about Difference Between Sn1 And Sn2 Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.