Difference Between Molecule And Compound With Examples
Juapaving
Mar 17, 2025 · 6 min read
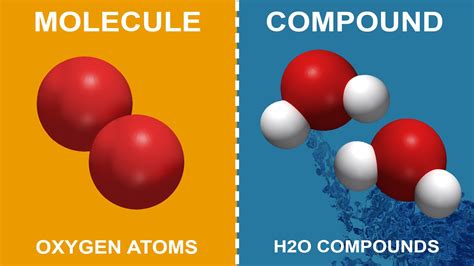
Table of Contents
The Difference Between Molecules and Compounds: A Deep Dive with Examples
Understanding the difference between molecules and compounds is fundamental to grasping the basics of chemistry. While the terms are often used interchangeably, they represent distinct concepts with subtle yet crucial differences. This article will delve into the definitions of molecules and compounds, highlight their key distinctions, provide numerous examples, and explore some common misconceptions. We’ll also look at how these concepts relate to other chemical terms like atoms and elements.
What is a Molecule?
A molecule is defined as two or more atoms chemically bonded together. These atoms can be of the same element or different elements. The crucial aspect is the presence of a chemical bond, indicating a strong attractive force holding the atoms together. This bond involves the sharing or transfer of electrons between the atoms.
Key Characteristics of Molecules:
- Chemical Bonding: The defining feature of a molecule is the presence of chemical bonds between its constituent atoms. These bonds can be covalent (electron sharing) or, less commonly in the context of molecules, coordinate covalent (a special type of covalent bond). Ionic bonds (electron transfer) are typically associated with compounds (explained later).
- Discrete Units: Molecules exist as distinct, independent units. They are not indefinitely extended in space like some crystals or ionic compounds.
- Neutral Charge: Generally, molecules carry a neutral electrical charge. However, there are exceptions, such as polyatomic ions (discussed further below).
- Specific Geometry: The atoms within a molecule are arranged in a specific three-dimensional geometry dictated by the types of bonds and the electron repulsion between atoms. This geometry influences the molecule's properties.
Examples of Molecules:
- Oxygen (O₂): Two oxygen atoms covalently bonded. This is a diatomic molecule, meaning it consists of two atoms of the same element.
- Nitrogen (N₂): Similar to oxygen, this is a diatomic molecule composed of two nitrogen atoms.
- Water (H₂O): Two hydrogen atoms covalently bonded to one oxygen atom. This is a triatomic molecule containing atoms of different elements.
- Methane (CH₄): One carbon atom covalently bonded to four hydrogen atoms.
- Glucose (C₆H₁₂O₆): A complex molecule composed of carbon, hydrogen, and oxygen atoms. Glucose is a vital sugar in biological systems.
- DNA (Deoxyribonucleic Acid): An incredibly large and complex molecule crucial for life, made of nucleotides linked together.
What is a Compound?
A compound is a substance formed when two or more different elements are chemically bonded together in fixed proportions. Crucially, a compound has properties different from the elements that make it up. This distinct set of properties arises from the chemical interaction between the elements.
Key Characteristics of Compounds:
- Different Elements: The defining feature of a compound is the presence of at least two different elements.
- Fixed Proportions: The elements within a compound are always present in a specific ratio, as dictated by their chemical formula. For instance, water (H₂O) always has a 2:1 ratio of hydrogen to oxygen atoms.
- New Properties: Compounds have unique physical and chemical properties distinct from those of their constituent elements. For example, sodium (Na) is a highly reactive metal, and chlorine (Cl) is a poisonous gas. However, their combination forms sodium chloride (NaCl), or table salt, a relatively inert and edible substance.
- Chemical Formulas: Compounds are represented by chemical formulas, which indicate the types and numbers of atoms in a molecule of the compound.
Examples of Compounds:
- Water (H₂O): As mentioned before, water is a compound because it consists of two different elements, hydrogen and oxygen, chemically bonded together.
- Sodium Chloride (NaCl): Table salt, a compound composed of sodium and chlorine.
- Carbon Dioxide (CO₂): A compound essential for plant life, comprising carbon and oxygen.
- Sulfuric Acid (H₂SO₄): A highly corrosive compound used in various industrial processes.
- Ethanol (C₂H₅OH): A common alcohol found in alcoholic beverages.
The Relationship Between Molecules and Compounds
The relationship between molecules and compounds is often a source of confusion. Here's a clear breakdown:
-
All compounds are molecules, but not all molecules are compounds. This is the most important distinction. A molecule simply signifies a group of atoms chemically bound together. A compound is a specific type of molecule where those atoms represent at least two different elements.
-
Diatomic molecules are molecules but not compounds: Elements like oxygen (O₂) and nitrogen (N₂) exist as diatomic molecules. They are molecules because they consist of bonded atoms, but they aren't compounds because they only contain one type of element.
-
Polyatomic ions are charged molecules: These molecules carry an overall electrical charge due to an imbalance in the number of protons and electrons. Examples include the hydroxide ion (OH⁻) and the sulfate ion (SO₄²⁻). Though charged, they are still molecules because they are composed of covalently bonded atoms. They can form ionic compounds with other ions.
-
Network covalent solids aren't considered molecules in the traditional sense: Materials like diamond (pure carbon) and quartz (SiO₂) have extensive networks of covalently bonded atoms. While they are formed from covalent bonds, they don't exist as discrete molecules. They extend indefinitely in all directions.
Common Misconceptions
-
Mixtures are not compounds: A mixture is a physical combination of substances, not a chemical one. The components of a mixture retain their individual properties, unlike compounds, where new properties emerge. Saltwater is a mixture, not a compound.
-
All ionic compounds are compounds, but not all compounds are ionic: Ionic compounds are formed by the electrostatic attraction between oppositely charged ions (cations and anions). While many compounds are ionic, many others are covalent (formed by shared electrons).
Advanced Concepts: Isomers and allotropes
To further solidify the understanding of molecules and compounds, let's examine isomers and allotropes:
-
Isomers: These are molecules with the same molecular formula but different structural arrangements. For example, glucose and fructose both have the formula C₆H₁₂O₆, but their atoms are arranged differently, leading to different properties. Isomerism is prevalent in organic chemistry.
-
Allotropes: These are different structural forms of the same element. A classic example is carbon: it can exist as diamond (a strong, hard material), graphite (a soft, slippery material), and fullerenes (spherical or tubular structures). Allotropes represent different ways the atoms of a single element can bond to each other.
Conclusion
The distinctions between molecules and compounds, though subtle, are crucial in chemistry. Understanding these distinctions, along with related concepts like isomers and allotropes, provides a solid foundation for exploring the complexities of chemical structures and their properties. Remember: all compounds are molecules, but not all molecules are compounds. The presence of different elements and the emergence of unique properties distinguish compounds from other types of molecules. By mastering these definitions and examples, you'll have a better grasp of the fundamental building blocks of matter.
Latest Posts
Latest Posts
-
How Many Sides Are In A Parallelogram
Mar 17, 2025
-
How To Change A Ratio Into A Percentage
Mar 17, 2025
-
Common Denominator Of 3 And 4
Mar 17, 2025
-
A Man Went Door To Door Posing As A Goldsmith
Mar 17, 2025
-
Coefficient Of Linear Expansion And Volume Expansion
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Difference Between Molecule And Compound With Examples . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
