Difference Between Molar Mass And Molecular Mass
Juapaving
Mar 21, 2025 · 6 min read
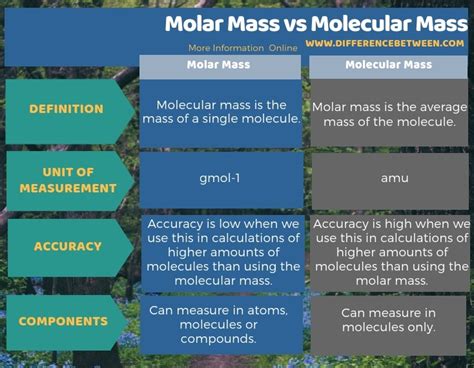
Table of Contents
Delving into the Differences: Molar Mass vs. Molecular Mass
Understanding the fundamental concepts in chemistry is crucial for anyone navigating the world of molecules and compounds. Two terms often causing confusion are molar mass and molecular mass. While closely related, they represent distinct concepts with different applications. This comprehensive guide will illuminate the differences between these two crucial terms, clarifying their definitions, units, and applications. We will explore their significance in stoichiometry, chemical reactions, and various scientific fields.
Defining Molecular Mass
Molecular mass, also known as molecular weight, refers to the mass of a single molecule of a substance. It's calculated by summing the atomic masses of all the atoms present in a single molecule. The atomic mass of an element is the average mass of all its isotopes, weighted by their natural abundance. Therefore, molecular mass is an average mass, reflecting the natural isotopic distribution of the elements within the molecule.
Calculating Molecular Mass
Calculating molecular mass is a straightforward process. Consider the example of water (H₂O):
- Hydrogen (H): Atomic mass ≈ 1.008 amu (atomic mass units)
- Oxygen (O): Atomic mass ≈ 15.999 amu
The molecular mass of water is calculated as follows: (2 × 1.008 amu) + 15.999 amu = 18.015 amu. This means a single water molecule has an average mass of approximately 18.015 amu.
Units of Molecular Mass
The standard unit for molecular mass is the atomic mass unit (amu), also known as the dalton (Da). While amu is commonly used, Da is becoming increasingly prevalent, especially in biochemistry and related fields.
Understanding Molar Mass
Molar mass, also known as molecular weight, represents the mass of one mole of a substance. A mole is a fundamental unit in chemistry, defined as the amount of a substance that contains as many elementary entities (atoms, molecules, ions, etc.) as there are atoms in 12 grams of carbon-12. This number, known as Avogadro's number, is approximately 6.022 x 10²³.
Calculating Molar Mass
The molar mass of a substance is numerically equivalent to its molecular mass, but the units differ. While molecular mass is expressed in amu, molar mass is expressed in grams per mole (g/mol). This means that the molar mass of water (H₂O) is 18.015 g/mol. This indicates that one mole of water molecules weighs 18.015 grams.
The Significance of the Mole
The concept of the mole is critical because it provides a bridge between the microscopic world of atoms and molecules and the macroscopic world of laboratory measurements. It allows chemists to work with easily measurable quantities of substances, relating them to the number of particles involved in chemical reactions.
Key Differences: A Comparative Table
| Feature | Molecular Mass | Molar Mass |
|---|---|---|
| Definition | Mass of a single molecule | Mass of one mole of a substance |
| Unit | Atomic mass unit (amu) or Dalton (Da) | Grams per mole (g/mol) |
| Magnitude | Typically small (in amu) | Typically large (in grams) |
| Representation | Represents the mass of an individual molecule | Represents the mass of a macroscopic quantity |
| Application | Useful in understanding individual molecules | Essential for stoichiometric calculations |
Applications in Stoichiometry and Chemical Reactions
Molar mass is indispensable in stoichiometric calculations, which involve determining the amounts of reactants and products in chemical reactions. For example, to determine the mass of a reactant needed to produce a specific amount of product, or to calculate the yield of a reaction, you need the molar mass of the substances involved.
Let's consider the balanced chemical equation for the combustion of methane:
CH₄ + 2O₂ → CO₂ + 2H₂O
Suppose we want to calculate the mass of oxygen (O₂) required to react completely with 10 grams of methane (CH₄).
- Find the molar mass:
- Molar mass of CH₄: (12.01 g/mol) + (4 × 1.008 g/mol) = 16.042 g/mol
- Molar mass of O₂: 2 × 15.999 g/mol = 31.998 g/mol
- Convert grams of CH₄ to moles:
Moles of CH₄ = (10 g CH₄) / (16.042 g/mol CH₄) ≈ 0.623 moles CH₄
- Use the stoichiometric ratio:
From the balanced equation, 1 mole of CH₄ reacts with 2 moles of O₂. Therefore:
Moles of O₂ = 0.623 moles CH₄ × (2 moles O₂ / 1 mole CH₄) = 1.246 moles O₂
- Convert moles of O₂ to grams:
Mass of O₂ = 1.246 moles O₂ × 31.998 g/mol O₂ ≈ 39.86 grams O₂
This example illustrates how molar mass is crucial for converting between mass and moles, a fundamental step in stoichiometric calculations. Molecular mass, on the other hand, wouldn't be directly useful in this type of calculation.
Molar Mass and Molecular Mass of Ionic Compounds
The concept of molecular mass is less applicable to ionic compounds because they don't exist as discrete molecules. Instead, they form crystal lattices with repeating units of ions. However, we can still define a formula mass for ionic compounds, which represents the sum of the atomic masses of all the atoms in the empirical formula of the compound. The molar mass of an ionic compound is then the formula mass expressed in grams per mole.
For example, for sodium chloride (NaCl):
- Atomic mass of Na: ≈ 22.99 amu
- Atomic mass of Cl: ≈ 35.45 amu
Formula mass of NaCl: 22.99 amu + 35.45 amu = 58.44 amu
Molar mass of NaCl: 58.44 g/mol
This means one mole of NaCl weighs 58.44 grams.
Beyond Stoichiometry: Applications in Various Fields
The concepts of molar mass and molecular mass extend far beyond stoichiometric calculations. They play vital roles in numerous scientific disciplines:
-
Biochemistry: Molecular mass is essential in characterizing proteins, nucleic acids, and other biological macromolecules. Techniques like mass spectrometry rely on precise mass measurements to identify and analyze these molecules. Molar mass is crucial in determining concentrations of biomolecules in solutions.
-
Polymer Chemistry: Molar mass is a critical parameter in polymer science, as it dictates the properties of polymers. The distribution of molar masses within a polymer sample affects its physical and mechanical characteristics.
-
Environmental Science: Determining the molar mass of pollutants is crucial for environmental monitoring and risk assessment. Understanding the molar mass of substances helps in calculating their concentrations in various environmental matrices (air, water, soil).
-
Material Science: The molar mass of components in materials influences their properties, such as strength, conductivity, and reactivity. This knowledge helps in designing and developing new materials with desired characteristics.
-
Pharmaceutical Sciences: Molar mass is vital in drug development and formulation. It is used in calculating dosages, determining drug concentrations, and understanding drug interactions within the body.
Conclusion: Clarifying the Distinction
While both molar mass and molecular mass relate to the mass of a substance, they serve different purposes and have distinct units. Molecular mass describes the mass of a single molecule, typically expressed in amu. Molar mass, however, represents the mass of one mole of a substance and is expressed in g/mol. Molar mass is indispensable for stoichiometric calculations and has extensive applications in various scientific disciplines, making it a fundamental concept in chemistry and beyond. A clear understanding of the distinction between these two concepts is essential for anyone delving into the world of chemistry and its applications. This comprehensive guide aims to equip you with a solid understanding of these crucial terms and their importance in scientific endeavors.
Latest Posts
Latest Posts
-
What Is 17 25 As A Decimal
Mar 28, 2025
-
150 Is 25 Of What Number
Mar 28, 2025
-
4 1 Rounded To The Nearest Tenth
Mar 28, 2025
-
The Physical Expression Of A Gene Is Known As The
Mar 28, 2025
-
Which Of The Following Is A Homogeneous Mixture
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Difference Between Molar Mass And Molecular Mass . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
