Difference Between Electron And Molecular Geometry
Juapaving
Mar 14, 2025 · 5 min read
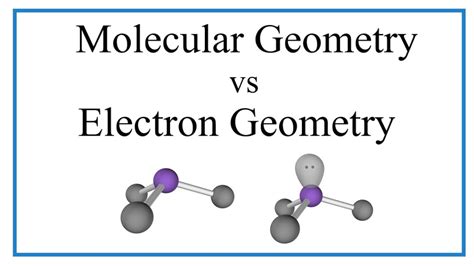
Table of Contents
Unveiling the Subtle Differences: Electron Geometry vs. Molecular Geometry
Understanding the three-dimensional arrangement of atoms within a molecule is crucial in chemistry. This understanding dictates the molecule's properties, reactivity, and overall behavior. Two closely related yet distinct concepts often cause confusion: electron geometry and molecular geometry. While both describe the spatial arrangement of atoms and electron pairs, they focus on different aspects, leading to potentially different outcomes. This article delves deep into the differences between electron and molecular geometry, providing clear explanations and examples to solidify your understanding.
What is Electron Geometry?
Electron geometry describes the arrangement of all electron pairs surrounding the central atom in a molecule, including both bonding pairs (involved in covalent bonds) and lone pairs (non-bonding electrons). It focuses solely on the electron repulsion, ignoring the types of electron pairs. The fundamental principle governing electron geometry is Valence Shell Electron Pair Repulsion (VSEPR) theory. VSEPR theory postulates that electron pairs around a central atom will arrange themselves to minimize repulsion, resulting in specific geometric shapes. This arrangement maximizes the distance between electron pairs, leading to the most stable configuration.
Key Factors Influencing Electron Geometry
- Number of electron pairs: The total number of electron pairs (bonding and lone pairs) around the central atom is the primary determinant of electron geometry.
- Repulsion between electron pairs: Lone pairs exert stronger repulsive forces than bonding pairs due to their closer proximity to the central atom. This difference in repulsion influences the bond angles and overall geometry.
Common Electron Geometries
- Linear: Two electron pairs arranged 180° apart.
- Trigonal planar: Three electron pairs arranged 120° apart in a single plane.
- Tetrahedral: Four electron pairs arranged 109.5° apart in a three-dimensional tetrahedron.
- Trigonal bipyramidal: Five electron pairs arranged in a trigonal bipyramidal shape with bond angles of 90° and 120°.
- Octahedral: Six electron pairs arranged 90° apart in an octahedron.
What is Molecular Geometry?
Molecular geometry, also known as molecular shape, describes the three-dimensional arrangement of only the atoms within a molecule. It considers the positions of the atoms bonded to the central atom, disregarding the lone pairs. While influenced by electron geometry, molecular geometry often differs due to the presence of lone pairs. Lone pairs, being more diffuse, occupy more space and exert stronger repulsive forces, pushing the bonding pairs closer together and altering the bond angles.
The Role of Lone Pairs in Molecular Geometry
Lone pairs are crucial in determining the difference between electron and molecular geometry. Because they don't bond with other atoms, their influence on the overall molecular shape is indirect but significant. They repel bonding pairs, distorting the ideal angles predicted by electron geometry. The stronger repulsion of lone pairs leads to a deviation from the ideal geometry, resulting in a different molecular shape.
Common Molecular Geometries
Molecular geometries often correspond to electron geometries when there are no lone pairs on the central atom. However, the presence of lone pairs modifies the shape significantly:
- Linear: Same as electron geometry.
- Bent or V-shaped: Derived from a trigonal planar electron geometry with one or two lone pairs.
- Trigonal pyramidal: Derived from a tetrahedral electron geometry with one lone pair.
- Tetrahedral: Same as electron geometry (no lone pairs).
- See-saw: Derived from a trigonal bipyramidal electron geometry with one or two lone pairs.
- T-shaped: Derived from a trigonal bipyramidal electron geometry with two lone pairs.
- Linear: Derived from a trigonal bipyramidal electron geometry with three lone pairs.
- Square pyramidal: Derived from an octahedral electron geometry with one lone pair.
- Square planar: Derived from an octahedral electron geometry with two lone pairs.
Key Differences Summarized
| Feature | Electron Geometry | Molecular Geometry |
|---|---|---|
| Focus | Arrangement of all electron pairs (bonding & lone pairs) | Arrangement of atoms only |
| Influence | VSEPR theory (electron repulsion) | VSEPR theory + influence of lone pairs |
| Lone Pairs | Included in determining the shape | Excluded; their influence affects the shape indirectly |
| Shape | Ideal shapes based on electron pair repulsion | Shape often deviates from ideal due to lone pair repulsion |
| Predictive Power | Predicts the overall spatial arrangement of electron density | Predicts the actual 3D arrangement of atoms |
Examples Illustrating the Difference
Let's illustrate the difference with some examples:
1. Methane (CH₄):
- Electron Geometry: Tetrahedral (four bonding pairs, no lone pairs).
- Molecular Geometry: Tetrahedral (same as electron geometry because there are no lone pairs).
2. Ammonia (NH₃):
- Electron Geometry: Tetrahedral (three bonding pairs, one lone pair).
- Molecular Geometry: Trigonal pyramidal (the lone pair pushes the bonding pairs closer together, distorting the tetrahedron).
3. Water (H₂O):
- Electron Geometry: Tetrahedral (two bonding pairs, two lone pairs).
- Molecular Geometry: Bent or V-shaped (the two lone pairs repel the bonding pairs strongly, causing a significant bend).
4. Carbon Dioxide (CO₂):
- Electron Geometry: Linear (two bonding pairs, no lone pairs).
- Molecular Geometry: Linear (same as electron geometry because there are no lone pairs).
5. Sulfur hexafluoride (SF₆):
- Electron Geometry: Octahedral (six bonding pairs, no lone pairs).
- Molecular Geometry: Octahedral (same as electron geometry because there are no lone pairs).
Predicting Electron and Molecular Geometry: A Step-by-Step Approach
Predicting the electron and molecular geometries involves these steps:
- Draw the Lewis structure: This shows the arrangement of atoms and valence electrons.
- Count the electron pairs around the central atom: Include both bonding and lone pairs.
- Determine the electron geometry: Use VSEPR theory to predict the arrangement based on the number of electron pairs (linear, trigonal planar, tetrahedral, etc.).
- Determine the molecular geometry: Consider the positions of the atoms only, taking into account the influence of lone pairs on bond angles and overall shape.
Conclusion: The Importance of Distinguishing Electron and Molecular Geometry
Understanding the distinction between electron and molecular geometry is essential for comprehending a molecule's properties. While electron geometry provides the overall picture of electron distribution, molecular geometry dictates the molecule's actual shape and its subsequent interactions with other molecules. This knowledge is crucial in various fields, including predicting reactivity, understanding physical properties like polarity, and interpreting spectroscopic data. Mastering these concepts provides a solid foundation for further advancements in organic chemistry, inorganic chemistry, physical chemistry, and biochemistry. By clearly differentiating these two concepts, one can move towards a deeper understanding of the intricate world of molecular structure and function. The ability to accurately predict and visualize these geometries is a cornerstone skill for any aspiring chemist.
Latest Posts
Latest Posts
-
What Is 1 5 Cm In Mm
May 09, 2025
-
How Does A Subsidy Affect Supply
May 09, 2025
-
What Is The Purpose Of A Contractile Vacuole
May 09, 2025
-
The Nuclear Membrane Reforms During Which Phase Of Mitosis
May 09, 2025
-
How To Calculate The Bandwidth Of A Signal
May 09, 2025
Related Post
Thank you for visiting our website which covers about Difference Between Electron And Molecular Geometry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.