Describe Rutherford's Model Of An Atom
Juapaving
Mar 31, 2025 · 6 min read
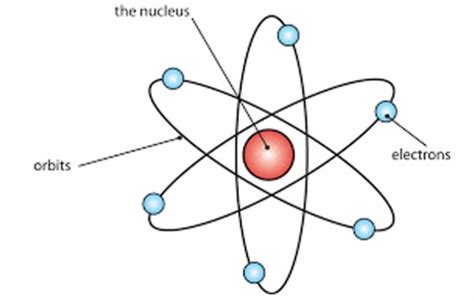
Table of Contents
Rutherford's Model of the Atom: A Deep Dive into the Nuclear Atom
Ernest Rutherford's groundbreaking gold foil experiment revolutionized our understanding of the atom, forever changing the scientific landscape. Before Rutherford, the prevailing model was the plum pudding model proposed by J.J. Thomson, which depicted the atom as a positively charged sphere with negatively charged electrons embedded within it, like plums in a pudding. Rutherford's experiment shattered this model and paved the way for a more accurate representation of atomic structure – the nuclear model of the atom. This article delves deep into Rutherford's model, exploring its experimental basis, key features, limitations, and lasting impact on the field of atomic physics.
The Gold Foil Experiment: A Revolutionary Breakthrough
Rutherford's experiment, conducted in 1909 by his students Hans Geiger and Ernest Marsden, involved bombarding a thin gold foil with alpha particles (positively charged helium nuclei). The expectation, based on Thomson's model, was that the alpha particles would pass through the foil with only minor deflections. However, the results were astonishing.
Unexpected Results: The Birth of the Nuclear Atom
While the majority of alpha particles did pass straight through, a small but significant number were deflected at large angles, and some even bounced straight back. This unexpected scattering pattern couldn't be explained by Thomson's model. The massive deflections suggested that the alpha particles were encountering a concentrated positive charge within the atom, a charge strong enough to repel the positively charged alpha particles.
Interpreting the Data: The Nuclear Model Emerges
To explain these observations, Rutherford proposed a revolutionary new model of the atom. He postulated that:
- The atom is mostly empty space: The fact that most alpha particles passed through undeflected indicated that the atom contains a vast amount of empty space.
- The atom possesses a tiny, dense, positively charged nucleus: The large-angle scattering of alpha particles implied the existence of a small, highly concentrated positive charge at the center of the atom, which he termed the nucleus. This nucleus contains almost all of the atom's mass.
- Electrons orbit the nucleus: To maintain electrical neutrality, Rutherford proposed that negatively charged electrons orbit the nucleus at a considerable distance. These electrons were thought to be in constant motion to prevent them from being pulled into the nucleus by electrostatic attraction.
Key Features of Rutherford's Nuclear Model
Rutherford's model introduced several critical concepts that fundamentally altered our understanding of atomic structure:
1. The Nucleus: The Atom's Core
The nucleus, the central core of the atom, is incredibly small compared to the overall size of the atom. It contains nearly all the atom's mass and all of its positive charge, due to the presence of protons (positively charged particles). The discovery of the neutron, a neutral particle also residing in the nucleus, came later.
2. Electron Orbitals: A Sea of Empty Space
The electrons occupy the space surrounding the nucleus, orbiting it at a significant distance. The vast majority of the atom's volume is empty space. This explains why most alpha particles passed straight through the gold foil in Rutherford's experiment.
3. Electrostatic Forces: Maintaining Atomic Stability (or so it seemed)
The model relied on the electrostatic attraction between the positively charged nucleus and the negatively charged electrons to hold the atom together. The electrons were envisioned as circling the nucleus like planets orbiting a sun, held in place by the electromagnetic force.
Limitations of Rutherford's Model
Despite its revolutionary nature, Rutherford's model had significant limitations, primarily concerning the stability of the atom:
1. Classical Physics Fails: The Instability Problem
According to classical physics, an orbiting electron should continuously emit electromagnetic radiation, losing energy in the process. This energy loss would cause the electron to spiral into the nucleus, leading to the collapse of the atom. This instability contradicts the observed stability of atoms.
2. No Explanation for Atomic Spectra
Rutherford's model could not explain the discrete nature of atomic spectra. When atoms are heated, they emit light at specific wavelengths. This discrete emission of light implies that the energy levels of electrons are quantized, meaning they can only exist at specific energy levels. Rutherford's model, however, provided no mechanism for explaining this quantization of energy.
3. No Description of Electron Arrangement
The model didn't provide any information about the arrangement or distribution of electrons around the nucleus. It simply suggested that they orbited the nucleus, without specifying their specific orbits or energy levels.
The Transition to the Bohr Model
The limitations of Rutherford's model were addressed by Niels Bohr in his improved model. Bohr incorporated the concept of quantization of energy, suggesting that electrons could only occupy specific orbits around the nucleus with defined energy levels. This successfully addressed the instability problem and explained the discrete nature of atomic spectra.
The Lasting Impact of Rutherford's Model
Despite its limitations, Rutherford's model remains a landmark achievement in atomic physics. Its key contributions include:
- Establishment of the nuclear atom: The model firmly established the concept of a central nucleus containing most of the atom's mass and positive charge, surrounded by orbiting electrons. This fundamentally changed our understanding of atomic structure.
- Foundation for future models: Rutherford's work laid the groundwork for subsequent models, such as the Bohr model and the quantum mechanical model, which refined and expanded upon his initial findings.
- Advancement of experimental techniques: The gold foil experiment demonstrated the power of experimental physics to probe the fundamental structure of matter.
Further Exploration: Isotopes and Nuclear Physics
Rutherford's work opened up the field of nuclear physics. His experiments laid the foundation for understanding isotopes, which are atoms of the same element with the same number of protons but a different number of neutrons. This discovery further expanded our comprehension of atomic structure and led to significant advancements in fields such as nuclear energy and nuclear medicine.
Conclusion: A Paradigm Shift in Atomic Physics
Ernest Rutherford's nuclear model of the atom represents a paradigm shift in our understanding of the fundamental building blocks of matter. Although it had limitations, it was a crucial step towards a more accurate and comprehensive understanding of atomic structure. The gold foil experiment remains a classic example of how ingenious experimental design can lead to revolutionary discoveries, fundamentally altering the scientific landscape. Rutherford's work continues to inspire generations of physicists, underscoring the enduring importance of his contribution to our understanding of the atom. The legacy of his model remains profound, influencing countless areas of science and technology. His contribution is a testament to the power of curiosity, rigorous experimentation, and innovative thinking in unraveling the mysteries of the universe. The story of Rutherford's model isn't just a scientific narrative; it's a compelling example of the human quest for knowledge and the transformative power of scientific discovery. The simple, yet profound, image of a dense, positively charged nucleus surrounded by orbiting electrons remains a cornerstone of modern atomic theory, a direct descendant of Rutherford's revolutionary insight.
Latest Posts
Latest Posts
-
Why Does Heat Not Transfer Through Solids By Convection
Apr 01, 2025
-
Solid Has Definite Shape And Volume
Apr 01, 2025
-
What Animal Lays Eggs Thats Not A Bird
Apr 01, 2025
-
Common Multiples Of 18 And 24
Apr 01, 2025
-
What Are The Factors Of 94
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Describe Rutherford's Model Of An Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
