Density Of Mercury In G Cm3
Juapaving
Mar 23, 2025 · 5 min read
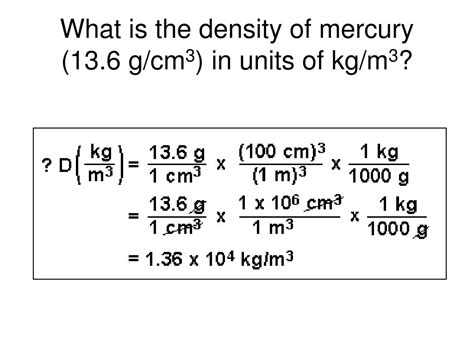
Table of Contents
Density of Mercury in g/cm³: A Comprehensive Guide
Mercury, a fascinating and often misunderstood element, holds a unique position in the periodic table. Its distinctive properties, particularly its exceptionally high density, make it a subject of scientific inquiry and practical applications across various fields. This article delves into the intricacies of mercury's density, exploring its value, the factors influencing it, and its significance in different contexts.
Understanding Density: A Fundamental Concept
Before diving into the specifics of mercury's density, let's establish a clear understanding of the concept itself. Density is a fundamental physical property that describes the mass of a substance per unit volume. It's essentially a measure of how tightly packed the atoms or molecules are within a given space. The standard unit for density is grams per cubic centimeter (g/cm³), although other units like kilograms per cubic meter (kg/m³) are also commonly used. The formula for calculating density is:
Density = Mass / Volume
This simple equation allows us to determine the density of any substance, provided we know its mass and volume.
The Density of Mercury: A Striking Feature
Mercury, with its chemical symbol Hg and atomic number 80, stands out for its unusually high density. At standard temperature and pressure (STP), which is typically defined as 0°C (273.15 K) and 1 atmosphere (atm), the density of mercury is approximately 13.534 g/cm³. This means that one cubic centimeter of mercury weighs 13.534 grams. This remarkably high density is a consequence of several factors, including its high atomic weight and strong metallic bonding.
Why is Mercury so Dense?
Several factors contribute to mercury's remarkably high density:
-
High Atomic Mass: Mercury has a relatively high atomic mass (200.59 u), meaning each atom is significantly heavier than atoms of many other elements. This directly contributes to its overall density.
-
Strong Metallic Bonding: Mercury atoms exhibit strong metallic bonding, resulting in a tightly packed structure. The close proximity of atoms contributes to the high mass concentration within a given volume.
-
Liquid State at Room Temperature: Unlike most metals, mercury is a liquid at room temperature. This liquid state allows for a more efficient packing of atoms compared to the more structured arrangements found in solid metals. However, its density is only slightly affected by temperature variations in its liquid state.
-
Relatively Small Atomic Radius: While its atomic mass is high, mercury's atomic radius is relatively small compared to other heavy metals. This compact size allows for a more efficient packing of atoms within a given space.
Factors Affecting Mercury Density
While the density of mercury at STP is relatively constant, certain factors can subtly influence its value:
-
Temperature: Temperature significantly affects the density of most substances, including mercury. As temperature increases, the volume of mercury expands, leading to a slight decrease in its density. This relationship is typically expressed through a temperature coefficient of density.
-
Pressure: Changes in pressure exert minimal effect on the density of mercury compared to its response to temperature. This is because mercury is an incompressible liquid. However, extremely high pressures might cause minute changes in its density.
-
Impurities: The presence of impurities in a mercury sample can slightly alter its density. Contaminants can increase or decrease the overall mass or volume, thus influencing the calculated density.
Applications Leveraging Mercury's High Density
The high density of mercury has led to its application in a variety of scientific instruments and industrial processes. Some notable examples include:
-
Barometers: Mercury's high density makes it ideal for barometers, which measure atmospheric pressure. The height of the mercury column in a barometer is directly proportional to the atmospheric pressure.
-
Manometers: Similar to barometers, manometers utilize mercury to measure pressure differences within closed systems. The difference in mercury column heights indicates the pressure differential.
-
Sphygmomanometers (Blood Pressure Gauges): Older models of sphygmomanometers, though increasingly replaced by digital equivalents, often employed mercury to measure blood pressure.
-
Thermometers: While mercury thermometers are increasingly phased out due to toxicity concerns, their high density and thermal expansion properties previously made them well-suited for temperature measurement.
-
Electrical Switches: Mercury's unique properties make it suitable for some specialized electrical switches, although concerns about its environmental impact have greatly reduced these applications.
-
Dental Amalgam: Mercury was historically used in dental fillings (amalgam) as a binder to combine with other metals. However, due to health and environmental concerns, this application is also declining.
Safety Precautions when Handling Mercury
Due to its toxicity and volatility, handling mercury requires stringent safety precautions. Mercury vapor is particularly hazardous, posing respiratory and neurological risks. It is crucial to:
-
Avoid Skin Contact: Mercury should never come into direct contact with skin. Gloves and appropriate protective clothing are essential.
-
Ensure Adequate Ventilation: Always work with mercury in well-ventilated areas to minimize exposure to mercury vapor.
-
Proper Disposal: Mercury waste should never be disposed of in regular trash. Special arrangements for the proper disposal of mercury-containing materials must be made to avoid environmental contamination.
-
Emergency Response: In case of mercury spills, immediate action should be taken to contain and clean up the spill according to established safety protocols.
The Future of Mercury's Applications
The increasing awareness of mercury's toxicity and its environmental impact has prompted a global push to reduce and eliminate its use wherever possible. Many applications once reliant on mercury are gradually transitioning to safer and more environmentally friendly alternatives. This shift reflects the prioritization of environmental protection and public health.
Conclusion
The density of mercury at 13.534 g/cm³ is a remarkable property that has shaped its historical applications in science and industry. However, the inherent toxicity of mercury and the growing awareness of its environmental consequences are driving a significant shift away from its use in many applications. Understanding the properties of mercury, including its density, is crucial for both appreciating its historical importance and making informed decisions about its responsible use and disposal in the future. Continued research and the development of safer alternatives will play a vital role in shaping the future of mercury's applications.
Latest Posts
Latest Posts
-
Blank Is The Process By Which An Organism Produces Offspring
Mar 25, 2025
-
Is Tap Water A Pure Substance Or A Mixture
Mar 25, 2025
-
46 Inches Is How Many Feet
Mar 25, 2025
-
How Many Feet Is 30 Yards
Mar 25, 2025
-
Life Cycle Of A Silk Moth
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Density Of Mercury In G Cm3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
