Delta H Is Negative Exothermic Or Endothermic
Juapaving
Mar 15, 2025 · 6 min read
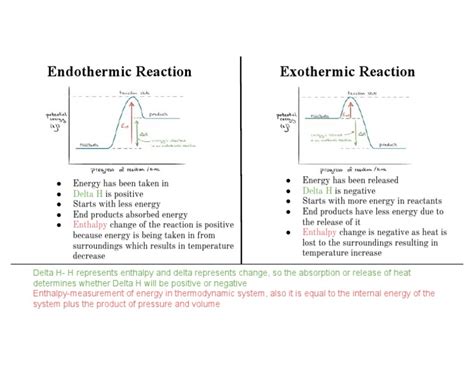
Table of Contents
Delta H is Negative: Exothermic or Endothermic? Understanding Enthalpy Change
The concept of enthalpy change (ΔH) is fundamental to understanding chemical reactions and their thermodynamic properties. Frequently, students encounter confusion surrounding the relationship between the sign of ΔH and whether a reaction is exothermic or endothermic. This comprehensive guide will clarify this crucial concept, exploring the meaning of ΔH, the distinctions between exothermic and endothermic processes, and providing numerous examples to solidify your understanding.
Understanding Enthalpy (H) and Enthalpy Change (ΔH)
Before delving into the specifics of ΔH being negative, let's first establish a clear understanding of enthalpy itself. Enthalpy (H) is a thermodynamic property representing the total heat content of a system at constant pressure. It's a state function, meaning its value depends only on the current state of the system and not on the path taken to reach that state. We can't directly measure enthalpy, but we can measure changes in enthalpy.
Enthalpy change (ΔH) signifies the difference in enthalpy between the products and reactants of a chemical reaction or physical process. It's calculated as:
ΔH = H<sub>products</sub> - H<sub>reactants</sub>
This equation reveals that:
- A negative ΔH: Indicates that the enthalpy of the products is lower than the enthalpy of the reactants. Energy is released during the process.
- A positive ΔH: Indicates that the enthalpy of the products is higher than the enthalpy of the reactants. Energy is absorbed during the process.
Exothermic Reactions: ΔH < 0
An exothermic reaction is a process that releases heat to its surroundings. In exothermic reactions, the system's enthalpy decreases, resulting in a negative ΔH. The energy released is often manifested as heat, causing a temperature increase in the surroundings. Think of it like this: the system is losing energy, and that energy is transferred to the environment.
Key Characteristics of Exothermic Reactions:
- Negative ΔH: This is the defining characteristic.
- Heat is released: The surroundings become warmer.
- Products have lower enthalpy than reactants: The system loses energy.
- Often spontaneous (but not always): Many exothermic reactions occur naturally without external intervention.
Examples of Exothermic Reactions:
- Combustion: Burning fuels like wood, propane, or gasoline. These reactions release a significant amount of heat. The combustion of methane, for instance: CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(l) has a highly negative ΔH.
- Neutralization reactions: The reaction between an acid and a base. The heat generated can be significant, especially with strong acids and bases. For example, the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH): HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l) is exothermic.
- Formation of many ionic compounds: The formation of ionic bonds from their constituent ions releases a considerable amount of energy. The formation of sodium chloride from sodium and chlorine atoms is a classic example.
- Condensation: The phase transition from gas to liquid, as in the condensation of water vapor. The molecules release energy as they form stronger intermolecular forces.
Endothermic Reactions: ΔH > 0
An endothermic reaction is a process that absorbs heat from its surroundings. In endothermic reactions, the system's enthalpy increases, resulting in a positive ΔH. The energy absorbed is often used to break bonds within the reactants, leading to a decrease in the surrounding temperature. Think of it as the system gaining energy from its environment.
Key Characteristics of Endothermic Reactions:
- Positive ΔH: This is the defining characteristic.
- Heat is absorbed: The surroundings become cooler.
- Products have higher enthalpy than reactants: The system gains energy.
- Often non-spontaneous (but not always): Many endothermic reactions require external energy input to proceed.
Examples of Endothermic Reactions:
- Melting of ice: The phase transition from solid to liquid requires energy input to break the hydrogen bonds holding the water molecules in a rigid structure.
- Photosynthesis: Plants absorb energy from sunlight to convert carbon dioxide and water into glucose and oxygen. This process requires a significant energy input.
- Decomposition reactions: Many decomposition reactions require heat input to break down compounds into simpler substances. For example, the decomposition of calcium carbonate (CaCO₃) into calcium oxide (CaO) and carbon dioxide (CO₂) requires heat.
- Dissolving certain salts in water: Some salts, like ammonium nitrate (NH₄NO₃), absorb heat when dissolved in water, causing the solution to cool down. This is commonly used in cold packs.
Why the Confusion? Focus on the System, Not the Surroundings
The primary source of confusion stems from focusing on the temperature change of the surroundings rather than the enthalpy change of the system. Exothermic reactions release heat into the surroundings, making them warmer, while endothermic reactions absorb heat from the surroundings, making them cooler. However, the sign of ΔH always refers to the change in enthalpy within the system undergoing the reaction or process. A negative ΔH always means an exothermic reaction, irrespective of the temperature change observed in the surroundings.
Visualizing Enthalpy Changes with Energy Diagrams
Energy diagrams provide a visual representation of enthalpy changes during a reaction. In an exothermic reaction, the products are at a lower energy level than the reactants, indicating energy release. In an endothermic reaction, the products are at a higher energy level, signifying energy absorption.
(Insert here a simple energy diagram illustrating an exothermic reaction with products at a lower energy level than reactants and a second diagram illustrating an endothermic reaction with products at a higher energy level than reactants. Labels should clearly indicate ΔH and the relative energy levels of reactants and products.)
Calculating ΔH: Hess's Law and Standard Enthalpies of Formation
Determining the enthalpy change for a reaction experimentally can be challenging. Hess's Law offers a powerful alternative. It states that the total enthalpy change for a reaction is independent of the pathway taken. This means that if a reaction can be expressed as a sum of multiple steps, the overall enthalpy change is the sum of the enthalpy changes for each step.
Standard enthalpies of formation (ΔH<sub>f</sub>°) provide another approach. The standard enthalpy of formation is the enthalpy change when one mole of a compound is formed from its constituent elements in their standard states (usually at 298K and 1 atm). Using these values, we can calculate the ΔH for a reaction using the following equation:
ΔH°<sub>rxn</sub> = Σ[ΔH<sub>f</sub>°(products)] - Σ[ΔH<sub>f</sub>°(reactants)]
This equation provides a convenient method for predicting enthalpy changes without performing experiments.
Practical Applications of Enthalpy Change
Understanding ΔH has far-reaching applications in various fields:
- Chemical Engineering: Designing and optimizing industrial processes, such as refining petroleum or manufacturing chemicals, requires precise control of enthalpy changes to ensure efficient energy use and safety.
- Materials Science: Studying the formation of materials and alloys relies on knowledge of enthalpy changes to predict stability and properties.
- Environmental Science: Assessing the environmental impact of processes, like combustion or waste disposal, necessitates understanding the enthalpy changes involved.
- Medicine and Biology: Metabolic processes, such as respiration and digestion, are governed by enthalpy changes, influencing energy production and storage in living organisms.
Conclusion
The sign of ΔH unequivocally determines whether a reaction is exothermic (ΔH < 0) or endothermic (ΔH > 0). Remember to focus on the enthalpy change within the system rather than the temperature change of the surroundings. Understanding this crucial concept opens doors to a deeper comprehension of chemical reactions, thermodynamics, and their wide-ranging applications in diverse fields. By grasping the principles outlined above, including Hess's Law and standard enthalpies of formation, you'll be well-equipped to analyze and predict enthalpy changes with confidence.
Latest Posts
Latest Posts
-
What Is 40 Percent Of 150
Mar 15, 2025
-
What Are The Common Multiples Of 9 And 12
Mar 15, 2025
-
How Tall Is 62 Inches In Feet
Mar 15, 2025
-
Which Of The Following Are Correctly Matched
Mar 15, 2025
-
Whats The Square Root Of X
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Delta H Is Negative Exothermic Or Endothermic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
