C2h6 + O2 Co2 + H2o
Juapaving
May 09, 2025 · 5 min read
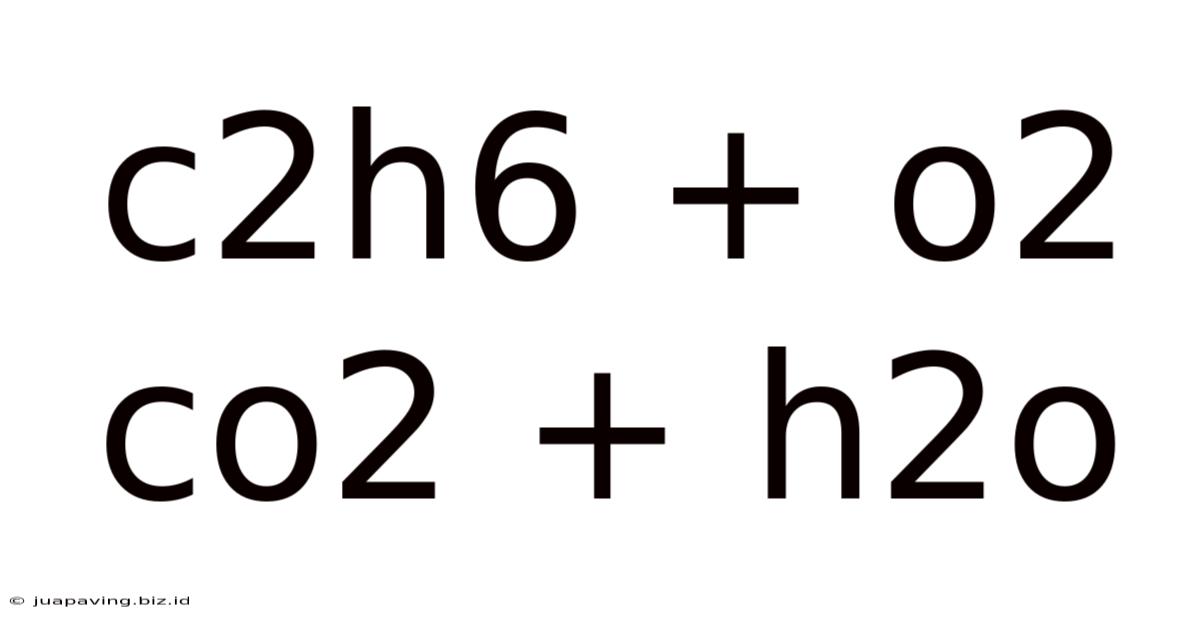
Table of Contents
Combustion of Ethane: A Deep Dive into C2H6 + O2 → CO2 + H2O
The chemical equation C₂H₆ + O₂ → CO₂ + H₂O represents the combustion of ethane, a crucial reaction with significant implications in various fields, from industrial processes to environmental science. This seemingly simple equation hides a wealth of complexity regarding stoichiometry, thermodynamics, kinetics, and environmental impact. This article will delve deep into each aspect, providing a comprehensive understanding of this fundamental chemical process.
Understanding the Basics: Stoichiometry and Balanced Equations
Before delving into the intricacies, let's ensure we have a solid foundation. The unbalanced equation C₂H₆ + O₂ → CO₂ + H₂O is insufficient for accurate calculations and analysis. A balanced equation accurately reflects the law of conservation of mass, ensuring the number of atoms of each element remains constant throughout the reaction.
The balanced equation for the complete combustion of ethane is:
2C₂H₆ + 7O₂ → 4CO₂ + 6H₂O
This equation tells us that two molecules of ethane react with seven molecules of oxygen to produce four molecules of carbon dioxide and six molecules of water. This ratio is crucial for determining the amounts of reactants needed and products formed in any given reaction. Understanding stoichiometry is the cornerstone of analyzing and predicting the outcomes of combustion reactions.
Mole Ratios and Calculations
The balanced equation provides the molar ratios. For instance, for every 2 moles of ethane consumed, 7 moles of oxygen are required, and 4 moles of carbon dioxide and 6 moles of water are produced. These ratios are fundamental for performing stoichiometric calculations, allowing us to determine the amount of product formed from a given amount of reactant or vice versa. This is crucial in industrial settings where precise control over reactant quantities is essential for efficient and safe operation.
Thermodynamics of Ethane Combustion: Energy Considerations
Combustion reactions are exothermic, meaning they release heat. The combustion of ethane is highly exothermic, releasing a considerable amount of energy. This energy release is quantifiable through thermodynamic parameters such as enthalpy change (ΔH). The enthalpy change for the complete combustion of ethane is a large negative value, indicating a significant release of heat. This heat release is harnessed in various applications.
Heat of Combustion and its Applications
The heat of combustion, often expressed in kJ/mol or kcal/mol, represents the amount of heat released when one mole of a substance undergoes complete combustion under standard conditions. The high heat of combustion of ethane makes it a valuable fuel source. This energy is harnessed in various applications, including:
- Power generation: Ethane is used in power plants to generate electricity through combustion turbines.
- Industrial heating: Its high heat content makes it suitable for industrial processes requiring high temperatures.
- Heating homes: In some regions, ethane is used as a fuel source for home heating systems.
Kinetics of Ethane Combustion: Reaction Rate and Mechanisms
The rate at which the combustion reaction proceeds is determined by its kinetics. Several factors influence the reaction rate, including:
- Temperature: Higher temperatures generally lead to faster reaction rates.
- Concentration of reactants: Higher concentrations of ethane and oxygen increase the frequency of collisions between reactant molecules, leading to a faster reaction rate.
- Presence of catalysts: Catalysts can lower the activation energy, accelerating the reaction rate.
- Surface area: In heterogeneous combustion (e.g., burning ethane gas on a solid surface), the surface area available for the reaction significantly affects the rate.
Reaction Mechanisms: A Complex Process
The combustion of ethane isn't a single-step reaction. It involves a complex series of elementary steps, including initiation, propagation, and termination. These steps involve the formation of free radicals—highly reactive species with unpaired electrons—which contribute to the chain reaction nature of the combustion process. Understanding these mechanisms is crucial for optimizing combustion efficiency and controlling pollutant formation.
Environmental Impact of Ethane Combustion: Pollutants and Mitigation
While ethane combustion provides valuable energy, it also generates pollutants. Complete combustion ideally yields only carbon dioxide and water. However, incomplete combustion, due to insufficient oxygen or low temperatures, can lead to the formation of harmful pollutants, such as:
- Carbon monoxide (CO): A toxic gas that can lead to health problems.
- Unburnt hydrocarbons (UHCs): Contribute to smog formation and respiratory issues.
- Nitrogen oxides (NOx): Contribute to acid rain and smog.
- Particulate matter (PM): Microscopic particles that can cause respiratory problems and other health issues.
Mitigation Strategies: Minimizing Environmental Impact
Various strategies can mitigate the environmental impact of ethane combustion:
- Optimizing combustion conditions: Ensuring sufficient oxygen supply and appropriate temperatures helps promote complete combustion, minimizing pollutant formation.
- Using advanced combustion technologies: Techniques like lean-burn combustion or catalytic converters can significantly reduce emissions.
- Developing alternative fuels: Research into cleaner and more sustainable fuels is ongoing.
Applications of Ethane Combustion: Industry and Beyond
Ethane combustion plays a crucial role in several industrial processes and applications:
- Petrochemical industry: Ethane is a valuable feedstock for producing ethylene, a crucial building block for various plastics and polymers. Ethylene production often involves high-temperature cracking of ethane, often relying on partial combustion techniques.
- Power generation: As mentioned earlier, ethane is a significant fuel source for power plants. Its high energy density and relatively clean combustion make it a valuable energy resource.
- Heating and cooling: Ethane can also be used in direct-fired heating and cooling systems.
Future Directions: Research and Innovation
Research continues to focus on improving the efficiency and reducing the environmental impact of ethane combustion. This includes:
- Developing more efficient combustion technologies: Research into advanced combustion systems aims to further minimize emissions and enhance energy conversion efficiency.
- Exploring alternative fuel sources: Developing renewable and sustainable alternatives to fossil fuels is crucial for reducing greenhouse gas emissions.
- Advanced modeling and simulation: Computational fluid dynamics (CFD) and other advanced modeling techniques are used to optimize combustion processes and design cleaner combustion systems.
Conclusion
The combustion of ethane, represented by the equation 2C₂H₆ + 7O₂ → 4CO₂ + 6H₂O, is a fundamental chemical reaction with significant industrial and environmental implications. Understanding its stoichiometry, thermodynamics, kinetics, and environmental impact is crucial for optimizing its use as a fuel source and minimizing its negative consequences. Ongoing research focuses on enhancing the efficiency of ethane combustion and developing sustainable alternatives. The continuous development of cleaner combustion technologies and the pursuit of alternative fuels will be vital for a sustainable future. The seemingly simple equation represents a complex and fascinating area of study with far-reaching consequences for energy production and environmental stewardship.
Latest Posts
Latest Posts
-
Nucleolus In Plant Or Animal Cells
May 09, 2025
-
The Ratio Of Atoms In Hcl Is
May 09, 2025
-
Which Of The Following Does Not Represent A Function
May 09, 2025
-
Difference Between Low Level And High Level Language
May 09, 2025
-
Which Of The Following Is Not True About Insulin
May 09, 2025
Related Post
Thank you for visiting our website which covers about C2h6 + O2 Co2 + H2o . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.