Buffer Region On A Titration Curve
Juapaving
Apr 06, 2025 · 6 min read
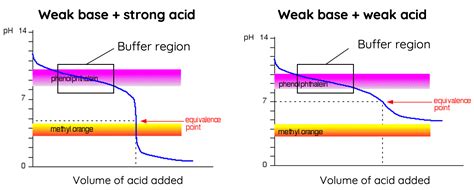
Table of Contents
Understanding the Buffer Region on a Titration Curve
The titration curve, a graphical representation of the pH change during a titration, offers invaluable insights into the properties of acids and bases. A key feature of many titration curves is the buffer region, a relatively flat section where the pH changes only gradually despite the addition of titrant. Understanding this region is crucial for comprehending buffer solutions and their applications in chemistry and biology. This article delves deep into the buffer region, explaining its characteristics, significance, and practical implications.
What is a Buffer Region?
The buffer region on a titration curve is characterized by a relatively small change in pH upon the addition of a significant amount of strong acid or strong base. This stability in pH is a hallmark of buffer solutions. A buffer solution resists changes in pH because it contains a weak acid and its conjugate base (or a weak base and its conjugate acid) in roughly equal concentrations. The buffer region typically spans approximately one pH unit centered around the pKa (or pKb) of the weak acid (or weak base).
Identifying the Buffer Region: A Visual Guide
On a titration curve, the buffer region is visually identifiable as a relatively flat portion of the curve. It's located before the equivalence point in the titration of a weak acid with a strong base, or before the equivalence point in the titration of a weak base with a strong acid. The steep rise or fall in pH occurs only after significant amounts of titrant have been added, indicating the exhaustion of the buffering capacity. The exact location and length of the buffer region depend on several factors, primarily the concentration of the weak acid/base and its pKa/pKb value.
The Chemistry Behind the Buffer Region
The buffering capacity stems from the equilibrium between the weak acid (HA) and its conjugate base (A⁻):
HA ⇌ H⁺ + A⁻
When a strong acid is added to the buffer solution, the added H⁺ ions react with the A⁻ ions to form more HA, minimizing the change in pH. Conversely, when a strong base is added, the added OH⁻ ions react with the HA to form more A⁻ and water, again minimizing the pH change. The equilibrium shifts to counteract the added acid or base, maintaining a relatively constant pH.
The Henderson-Hasselbalch Equation: A Quantitative Explanation
The Henderson-Hasselbalch equation provides a quantitative description of the relationship between the pH of a buffer solution, the pKa of the weak acid, and the concentrations of the weak acid and its conjugate base:
pH = pKa + log([A⁻]/[HA])
This equation demonstrates that when [A⁻] and [HA] are approximately equal (a ratio near 1), the pH of the buffer is approximately equal to the pKa of the weak acid. This condition corresponds to the midpoint of the buffer region. As the ratio [A⁻]/[HA] deviates significantly from 1 (either due to the addition of acid or base), the pH starts to change more dramatically, marking the boundary of the buffer region.
Factors Affecting the Buffer Region
Several factors influence the width and location of the buffer region:
- Concentration of the weak acid/base: Higher concentrations result in a wider buffer region, as more acid/base is available to neutralize added acid/base.
- pKa/pKb value: The buffer region is centered around the pKa (for weak acids) or pKb (for weak bases). A smaller pKa/pKb value results in a buffer region at a lower pH, and vice versa.
- Strength of the weak acid/base: A weaker acid/base will have a narrower buffer region compared to a stronger one.
Significance and Applications of the Buffer Region
The buffer region's significance lies in its ability to maintain a relatively stable pH, a crucial requirement in numerous chemical and biological systems. Understanding the buffer region is essential for:
1. Biological Systems
Biological systems are highly sensitive to pH changes. Many biological processes, such as enzyme activity and protein structure, depend on a carefully maintained pH. Buffers play a vital role in maintaining the pH of blood (maintained by the bicarbonate buffer system), intracellular fluids, and other biological compartments. The buffer region ensures that these systems can withstand small fluctuations in acid or base concentration without significant pH shifts, preventing potentially harmful consequences.
2. Chemical Reactions
Many chemical reactions require specific pH conditions to proceed efficiently. Buffer solutions are often used to maintain the desired pH during these reactions, ensuring optimal yields and preventing unwanted side reactions. The buffer region provides a stable pH environment, enabling precise control over reaction conditions.
3. Analytical Chemistry
In analytical chemistry, buffer solutions are essential for various techniques such as spectrophotometry and chromatography. Maintaining a constant pH is crucial for accurate and reproducible measurements. The buffer region guarantees that slight variations in the amount of titrant added will not significantly affect the pH and hence the experimental results.
4. Industrial Applications
Buffer solutions find wide applications in various industries, including pharmaceuticals, food processing, and cosmetics. Maintaining a specific pH is crucial for the stability and efficacy of many products. For example, buffers are used in the manufacturing of pharmaceuticals to maintain the stability of drug formulations and in the food industry to control the pH of food products and prevent spoilage.
Beyond the Buffer Region: Equivalence Point and Beyond
While the buffer region is a key aspect of a titration curve, it is crucial to understand the events occurring beyond this region.
Equivalence Point
The equivalence point is the point at which the moles of titrant added are stoichiometrically equal to the moles of analyte (the substance being titrated). At the equivalence point, the weak acid (or base) has been completely neutralized by the strong base (or acid). In the titration of a weak acid with a strong base, the equivalence point is characterized by a rapid pH increase; conversely, the equivalence point in the titration of a weak base with a strong acid is characterized by a rapid pH decrease. The location of the equivalence point provides information on the concentration of the unknown analyte.
Post-Equivalence Point
After the equivalence point, the pH changes rapidly because the solution is no longer buffered. The pH is primarily determined by the excess titrant. In the titration of a weak acid with a strong base, the pH is determined by the concentration of the excess hydroxide ions. In contrast, in the titration of a weak base with a strong acid, the pH is determined by the concentration of the excess hydronium ions.
Conclusion: The Importance of Understanding Buffer Regions
The buffer region on a titration curve is a critical concept in chemistry and beyond. Its understanding provides a foundation for comprehending buffer solutions and their significant role in various applications ranging from biological systems to industrial processes. The ability of buffers to resist pH changes, as depicted by the relatively flat buffer region, is essential for maintaining stable conditions in diverse environments. Understanding the factors affecting the buffer region's width and location, coupled with a knowledge of the equivalence point and post-equivalence point behavior, allows for effective utilization of buffer solutions in both research and practical settings. This comprehensive understanding underscores the importance of thoroughly grasping the buffer region within the context of acid-base titrations.
Latest Posts
Latest Posts
-
The Sum Of 5 Consecutive Odd Numbers Of 135
Apr 07, 2025
-
What Percent Of 50 Is 7
Apr 07, 2025
-
Words That Start With V For Kids
Apr 07, 2025
-
Packing Factor Of Bcc And Fcc
Apr 07, 2025
-
6 Quarts Of Water Is How Many Cups
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about Buffer Region On A Titration Curve . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
