Boiling Water Is A Chemical Or Physical Change
Juapaving
Apr 05, 2025 · 5 min read
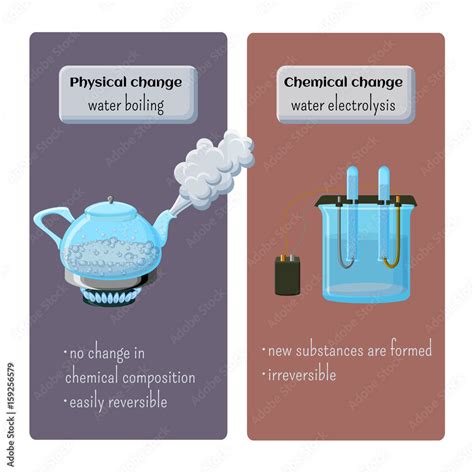
Table of Contents
Is Boiling Water a Chemical or Physical Change? A Deep Dive
The question of whether boiling water represents a chemical or physical change is a classic introductory chemistry conundrum. While seemingly simple, it delves into the fundamental principles of matter and its transformations. Understanding the difference between chemical and physical changes is crucial for grasping many scientific concepts. This article will explore the boiling of water in detail, examining the processes involved and definitively classifying the change. We'll also explore some related concepts and address common misconceptions.
Understanding Chemical vs. Physical Changes
Before we dive into the specifics of boiling water, let's establish a clear understanding of the difference between chemical and physical changes.
Physical changes alter the form or appearance of a substance but do not change its chemical composition. The molecules remain the same; only their arrangement or state of matter changes. Examples include melting ice, dissolving sugar in water, or cutting a piece of wood. The substance retains its fundamental chemical identity.
Chemical changes, also known as chemical reactions, involve the rearrangement of atoms and molecules to form new substances with different properties. These changes are often accompanied by observable indicators like a change in color, temperature, gas production, or the formation of a precipitate. Examples include burning wood, rusting iron, or cooking an egg. The original substance is fundamentally transformed into a new substance(s).
Analyzing the Boiling Process
When water boils, it transitions from a liquid state to a gaseous state (steam). Let's break down the process step-by-step:
The Role of Heat Energy
Heating water increases the kinetic energy of its molecules. These molecules are constantly in motion, and as their energy increases, they move faster and farther apart. This increased kinetic energy overcomes the intermolecular forces holding the water molecules together in the liquid phase.
Phase Transition: Liquid to Gas
At the boiling point (100°C or 212°F at standard atmospheric pressure), the kinetic energy of the water molecules is sufficient to overcome the attractive forces entirely. The water molecules escape the liquid phase and enter the gaseous phase, forming steam.
Molecular Structure Remains Intact
Crucially, during boiling, the water molecules themselves remain unchanged. Each water molecule (H₂O) retains its two hydrogen atoms and one oxygen atom. No new chemical bonds are formed, and no existing bonds are broken. The chemical composition of the water remains H₂O throughout the entire process.
Boiling Water: A Physical Change
Based on the analysis above, we can definitively conclude that boiling water is a physical change. The process involves a change in the state of matter (liquid to gas), but the chemical composition of the water remains the same. No new substances are formed. The water molecules simply gain enough energy to overcome their intermolecular attractions and move more freely in the gaseous phase.
Misconceptions Debunked
Several misconceptions often cloud the understanding of boiling water. Let's address some of the most common ones:
- The appearance of steam: Steam may appear different from liquid water, but this difference is only in its physical state, not its chemical makeup.
- Energy changes: While energy is added to the system during boiling, this energy alters the kinetic energy of the molecules, not their chemical structure.
- Bubbles: The formation of bubbles during boiling is a result of the water vapor forming and rising to the surface. This is a physical phenomenon, not a chemical reaction.
Expanding on Related Concepts
The boiling of water is a excellent example to illustrate several key concepts in chemistry and physics:
Heat Capacity and Specific Heat
The amount of heat required to raise the temperature of a substance is related to its heat capacity and specific heat. Water has a relatively high specific heat, meaning it requires a significant amount of energy to increase its temperature. This high specific heat is important for many biological and environmental processes.
Latent Heat of Vaporization
The energy required to convert a liquid into a gas at its boiling point is called the latent heat of vaporization. This energy is used to overcome the intermolecular forces holding the molecules in the liquid state, not to increase the kinetic energy of the molecules. For water, this latent heat is relatively high, requiring a considerable amount of energy to boil a given quantity of water.
Vapor Pressure and Boiling Point
The boiling point of a liquid is the temperature at which its vapor pressure equals the atmospheric pressure. At higher altitudes, where atmospheric pressure is lower, the boiling point of water is lower. This is why water boils at a lower temperature on mountaintops.
Superheating
Occasionally, water can be heated above its boiling point without boiling. This phenomenon, known as superheating, occurs when there are few nucleation sites (e.g., impurities or scratches in the container) for bubble formation. Once boiling starts, it will happen rapidly and violently. This is a safety hazard and demonstrates the importance of controlled heating.
The Critical Point
At extremely high temperatures and pressures, the distinction between liquid and gas disappears. This point is called the critical point, and for water, it is at approximately 374°C and 218 atmospheres of pressure. Above the critical point, water exists as a supercritical fluid, exhibiting properties of both liquids and gases.
Practical Applications and Everyday Examples
Understanding the physical nature of boiling water has numerous practical applications:
- Cooking: Boiling water is crucial for cooking various foods, ensuring proper sterilization and food safety.
- Cleaning: Boiling water can disinfect surfaces and remove germs effectively.
- Steam generation: Steam, produced by boiling water, is used for various purposes, including power generation, sterilization, and heating.
- Water purification: Boiling water is a simple and effective method to eliminate harmful bacteria and other microorganisms.
Conclusion: Boiling Water is a Physical Change
In summary, boiling water undergoes a physical change, not a chemical change. The process involves a transition of state from liquid to gas, driven by increased kinetic energy of water molecules. The chemical composition (H₂O) remains constant throughout the transformation. Understanding this fundamental distinction is crucial for a solid foundation in chemistry and physics, and it illuminates a wide variety of everyday processes and practical applications. While seemingly simple, the act of boiling water holds a surprising depth of scientific principles.
Latest Posts
Latest Posts
-
How Many Feet Are In 200 Inches
Apr 06, 2025
-
How Many Feet Are In A 100 Yards
Apr 06, 2025
-
Examples Of Abiotic Factors And Biotic Factors
Apr 06, 2025
-
For Every Action There Is An Equal Opposite Reaction
Apr 06, 2025
-
A Comparison Of Two Similar Quantities Using Division
Apr 06, 2025
Related Post
Thank you for visiting our website which covers about Boiling Water Is A Chemical Or Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
