Benedict's Reagent Tests For The Presence Of
Juapaving
Mar 13, 2025 · 6 min read
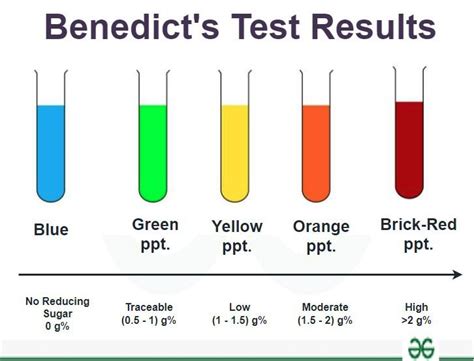
Table of Contents
Benedict's Reagent Tests for the Presence of Reducing Sugars: A Comprehensive Guide
Benedict's reagent is a widely used chemical reagent in chemistry and particularly in biochemistry to detect the presence of reducing sugars. This article will delve into the intricacies of Benedict's test, explaining its mechanism, applications, limitations, and interpretations. We will cover everything from the fundamental chemistry behind the reaction to practical considerations for conducting the test accurately.
Understanding Reducing Sugars
Before diving into the specifics of Benedict's reagent, let's clarify what constitutes a reducing sugar. Reducing sugars are carbohydrates that possess a free aldehyde (-CHO) or ketone (-C=O) group. This functional group can readily donate electrons, causing the reduction of another chemical species (like the copper in Benedict's reagent). Examples of reducing sugars include:
- Glucose: A crucial monosaccharide found in blood and a primary source of energy.
- Fructose: A monosaccharide found in fruits and honey.
- Galactose: A monosaccharide component of lactose (milk sugar).
- Lactose: A disaccharide composed of glucose and galactose.
- Maltose: A disaccharide composed of two glucose units.
Non-reducing sugars, on the other hand, lack a free aldehyde or ketone group, meaning they cannot donate electrons in the same way. Sucrose (table sugar) is a prime example of a non-reducing sugar because the anomeric carbons of both glucose and fructose are involved in the glycosidic bond, rendering the aldehyde and ketone groups unavailable for reduction.
The Chemistry of Benedict's Reagent
Benedict's reagent is an alkaline solution of copper(II) sulfate, sodium citrate, and sodium carbonate. The key component is the copper(II) sulfate (CuSO₄), which provides the copper ions that participate in the redox reaction. The sodium citrate acts as a chelating agent, preventing the precipitation of copper(II) hydroxide. Sodium carbonate provides the alkaline environment necessary for the reaction to occur.
When Benedict's reagent is heated with a solution containing a reducing sugar, a redox reaction takes place. The reducing sugar acts as a reducing agent, donating electrons to the copper(II) ions (Cu²⁺). This reduces the copper(II) ions to copper(I) ions (Cu⁺), which then precipitate out of solution as a reddish-brown copper(I) oxide (Cu₂O).
The overall reaction can be summarized as:
Reducing sugar + Cu²⁺ (blue) → Oxidized sugar + Cu⁺ (reddish-brown)
The intensity of the reddish-brown precipitate is directly proportional to the concentration of the reducing sugar in the solution. A small amount of reducing sugar will result in a green or yellow precipitate, while a large amount will produce a brick-red precipitate. The absence of a reducing sugar will leave the solution blue.
Performing the Benedict's Test: A Step-by-Step Guide
Conducting the Benedict's test is relatively straightforward, but accuracy is crucial for reliable results. Here's a detailed procedure:
- Prepare the Sample: Prepare a solution of the substance you wish to test. Ensure the sample is adequately dissolved in distilled water.
- Add Benedict's Reagent: Add approximately 1 ml of Benedict's reagent to 2 ml of the sample solution in a clean test tube.
- Heat the Mixture: Gently heat the test tube in a boiling water bath for 3-5 minutes. Avoid direct heating with a Bunsen burner, as this can lead to uneven heating and inaccurate results.
- Observe the Color Change: After heating, remove the test tube from the water bath and observe the color change. The color change will indicate the presence and concentration of reducing sugars.
Interpreting the Results
The color of the precipitate formed after heating provides crucial information about the presence and concentration of reducing sugars:
- Blue: No reducing sugar present. The solution remains the original blue color of Benedict’s reagent.
- Green: Very low concentration of reducing sugar.
- Yellow or Yellow-Green: Low concentration of reducing sugar.
- Orange: Moderate concentration of reducing sugar.
- Brick-red: High concentration of reducing sugar.
It's important to note that the intensity of the color change is qualitative rather than quantitative. While it indicates the presence and relative concentration, it doesn't provide precise numerical values. For precise quantification, other analytical techniques like spectrophotometry or chromatography are necessary.
Applications of Benedict's Test
Benedict's test finds applications in diverse fields, primarily focusing on the detection and quantification of reducing sugars:
- Clinical Diagnostics: Detecting glucose in urine (glycosuria) is a common application in medical diagnostics. Glycosuria can indicate conditions like diabetes mellitus. However, it's important to note that Benedict's test is a less precise method compared to blood glucose tests.
- Food Science: Identifying and quantifying reducing sugars in food products such as fruit juices, honey, and syrups. This helps in quality control and determining nutritional content.
- Biochemistry Research: Determining the presence of reducing sugars in biological samples, such as plant extracts or cell lysates, aids in studying carbohydrate metabolism and related pathways.
- Educational Purposes: Benedict's test is a valuable tool in educational settings to demonstrate redox reactions and the chemistry of carbohydrates.
Limitations of Benedict's Test
While Benedict's test is a simple and widely used method, it has several limitations:
- Lack of Specificity: The test detects all reducing sugars, not just glucose. Other reducing sugars can lead to false-positive results.
- Qualitative Nature: The test is qualitative, providing only an estimate of reducing sugar concentration. Precise quantitative measurements require more sophisticated techniques.
- Interference from Other Substances: Some substances can interfere with the reaction, leading to inaccurate results. For instance, ascorbic acid (vitamin C) can act as a reducing agent, causing a false-positive result.
- Sensitivity: Benedict's test is not as sensitive as some modern methods, meaning it might not detect very low concentrations of reducing sugars.
Comparison with Other Reducing Sugar Tests
Several other tests can detect reducing sugars, each with its own advantages and disadvantages. Some notable examples include:
- Barfoed's test: This test is more specific for monosaccharides than Benedict's test.
- Fehling's test: Similar to Benedict's test, but uses a different reagent formulation.
- Tollen's test: Uses silver nitrate and ammonia to detect aldehydes, including those in reducing sugars. This produces a silver mirror if positive.
Choosing the appropriate test depends on the specific application and the desired level of specificity and sensitivity.
Conclusion: A Valuable Tool in Biochemical Analysis
Benedict's reagent test remains a valuable and widely used method for detecting the presence of reducing sugars. Its simplicity, relatively low cost, and ease of use make it a popular choice in various settings. While limitations exist, primarily related to specificity and quantification, understanding these limitations and using the test appropriately ensures reliable results in many applications. Remember to always consider the context of the test, potential interferences, and interpret results cautiously. For precise quantification, more advanced analytical techniques are necessary. However, for a quick and relatively accurate indication of reducing sugar presence, Benedict's test remains a cornerstone of biochemical analysis.
Latest Posts
Latest Posts
-
5 Letter Words Beginning With Ps
May 09, 2025
-
How Do You Write 3 5 As A Percentage
May 09, 2025
-
What Happens When Light Goes Through A Prism
May 09, 2025
-
What Is The Importance Of The Start And Stop Codons
May 09, 2025
-
Images And Names Of Musical Instruments
May 09, 2025
Related Post
Thank you for visiting our website which covers about Benedict's Reagent Tests For The Presence Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.