Basic Building Block Of All Matter
Juapaving
Mar 13, 2025 · 6 min read
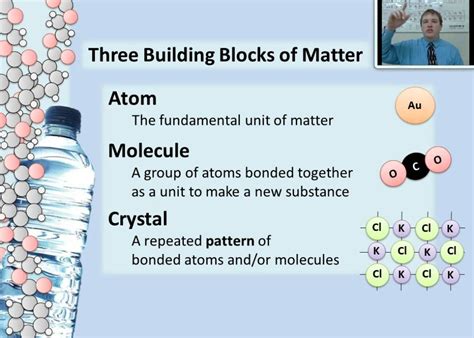
Table of Contents
The Basic Building Blocks of All Matter: A Deep Dive into Atoms and Subatomic Particles
The universe, in all its breathtaking complexity, is built from a surprisingly small set of fundamental components. Everything you see, touch, and experience – from the smallest grain of sand to the largest galaxy – is ultimately composed of the basic building blocks of matter. Understanding these building blocks is crucial to comprehending the nature of reality itself. This article delves into the fascinating world of atoms and subatomic particles, exploring their structure, properties, and interactions.
Atoms: The Foundation of Matter
For centuries, philosophers and scientists speculated about the fundamental nature of matter. The Greek philosopher Democritus proposed the concept of atomos, indivisible particles, but it wasn't until the early 20th century that the atomic theory gained widespread acceptance. Atoms are the smallest units of an element that retain the chemical properties of that element. They are incredibly tiny; a single grain of sand contains trillions upon trillions of atoms.
The Structure of an Atom: A Subatomic Symphony
Atoms are not solid, indivisible spheres as initially envisioned. Instead, they possess a complex internal structure comprised of three primary subatomic particles:
-
Protons: Positively charged particles located in the atom's nucleus. The number of protons determines the element's atomic number and its identity on the periodic table. For example, hydrogen has one proton, helium has two, and so on.
-
Neutrons: Neutrally charged particles also residing in the atom's nucleus. Neutrons contribute to the atom's mass but not its charge. The number of neutrons in an atom can vary, leading to different isotopes of the same element. Isotopes of an element have the same number of protons but different numbers of neutrons.
-
Electrons: Negatively charged particles that orbit the nucleus in electron shells or energy levels. Electrons are significantly lighter than protons and neutrons. The arrangement of electrons in these shells determines the atom's chemical behavior and how it interacts with other atoms to form molecules and compounds. The number of electrons typically equals the number of protons in a neutral atom, ensuring an overall neutral charge.
Atomic Number, Mass Number, and Isotopes: Key Distinctions
Understanding the following terms is vital to grasp atomic structure and behavior:
-
Atomic Number (Z): This is the number of protons in the nucleus of an atom. It uniquely identifies an element.
-
Mass Number (A): This represents the total number of protons and neutrons in the nucleus. It gives an indication of the atom's mass.
-
Isotopes: Atoms of the same element with the same atomic number but different mass numbers (due to varying numbers of neutrons). For example, carbon-12 and carbon-14 are isotopes of carbon. They have the same number of protons (6) but different numbers of neutrons (6 and 8 respectively). Some isotopes are stable, while others are radioactive, undergoing decay over time.
Delving Deeper: Subatomic Particles and the Standard Model
While the proton, neutron, and electron provide a basic understanding of atomic structure, they are not truly fundamental particles. These particles themselves are composed of even smaller, more fundamental constituents, governed by the Standard Model of particle physics.
Quarks: The Building Blocks of Protons and Neutrons
Protons and neutrons are not indivisible; they are made up of quarks. Quarks are fundamental particles that come in six different flavors or types: up, down, charm, strange, top, and bottom. Each quark also carries a fractional electric charge.
-
Protons: Consist of two up quarks and one down quark (uud).
-
Neutrons: Consist of one up quark and two down quarks (udd).
The strong force, mediated by gluons, binds quarks together to form hadrons, which include protons and neutrons.
Leptons: Electrons and Their Cousins
Electrons belong to a family of particles called leptons. Leptons are fundamental particles that do not experience the strong force. Besides electrons, other leptons include muons and tau particles, each with their corresponding neutrinos.
Gauge Bosons: The Force Carriers
The Standard Model also includes gauge bosons, which are force-carrying particles responsible for mediating fundamental interactions:
-
Photons: Mediate the electromagnetic force, responsible for interactions between charged particles.
-
Gluons: Mediate the strong force, binding quarks together within protons and neutrons.
-
W and Z bosons: Mediate the weak force, responsible for radioactive decay processes.
-
Graviton: A hypothetical particle that is believed to mediate the gravitational force, although it has not yet been directly observed.
Beyond the Standard Model: Open Questions and Future Research
The Standard Model has been incredibly successful in explaining a vast range of experimental observations in particle physics. However, it doesn't account for everything. Some of the open questions and areas of ongoing research include:
-
Dark Matter and Dark Energy: These mysterious substances make up the majority of the universe's mass-energy content, but their nature remains unknown.
-
Neutrino Mass: While neutrinos were initially believed to be massless, experiments have shown they possess a small but non-zero mass. The origin of this mass is still a puzzle.
-
The Hierarchy Problem: The Standard Model struggles to explain the vast difference in strength between the gravitational force and the other fundamental forces.
-
Grand Unified Theories (GUTs): Physicists are searching for a single theory that can unify the electromagnetic, weak, and strong forces.
-
Supersymmetry (SUSY): This theory proposes a symmetry between bosons and fermions, suggesting the existence of "superpartners" for known particles.
The Implications of Understanding Basic Building Blocks
Understanding the basic building blocks of matter is not just an academic pursuit; it has profound implications across various fields:
-
Materials Science: Knowledge of atomic and subatomic structures allows for the design and creation of new materials with specific properties, such as superconductors and advanced polymers.
-
Medicine: Medical imaging techniques like MRI and PET scans rely on the interactions of subatomic particles with the human body. Radiation therapy utilizes radioactive isotopes for cancer treatment.
-
Energy Production: Nuclear power plants utilize nuclear fission, a process involving the splitting of atomic nuclei, to generate electricity. Future fusion reactors aim to harness the energy released when atomic nuclei fuse together.
-
Technology: Semiconductors, the foundation of modern electronics, rely on the controlled manipulation of electrons in materials.
Conclusion: A Journey of Discovery Continues
The quest to understand the basic building blocks of all matter is a journey that continues to unfold. From the ancient Greek concept of atomos to the complexities of the Standard Model and beyond, our understanding has evolved dramatically. The ongoing exploration of the subatomic world promises further breakthroughs, revealing deeper secrets of the universe and leading to technological advancements that will shape our future. The journey into the heart of matter is a testament to human curiosity and our relentless pursuit of knowledge. Each new discovery builds upon previous understanding, pushing the boundaries of our comprehension and inspiring awe at the elegance and intricacy of the cosmos. The basic building blocks of matter, while seemingly simple in their fundamental nature, are the foundation upon which the entire universe, in all its vibrant complexity, is built.
Latest Posts
Latest Posts
-
Compare And Contrast A Light Microscope And An Electron Microscope
May 09, 2025
-
What Is 6 25 Meters In Feet And Inches
May 09, 2025
-
How Many Centimeters Are In 50 Millimeters
May 09, 2025
-
Describe The Epithelium Found In The Uterine Tube
May 09, 2025
-
What Animal Lay Eggs And Is Not A Bird
May 09, 2025
Related Post
Thank you for visiting our website which covers about Basic Building Block Of All Matter . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.