Balanced Chemical Equation For Magnesium Oxide
Juapaving
Apr 03, 2025 · 6 min read
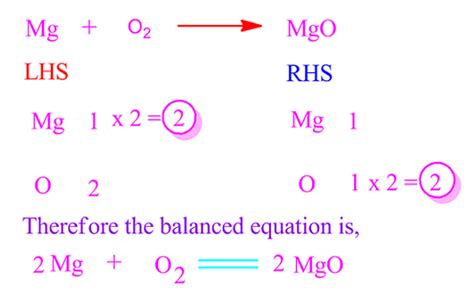
Table of Contents
- Balanced Chemical Equation For Magnesium Oxide
- Table of Contents
- The Balanced Chemical Equation for Magnesium Oxide: A Deep Dive
- The Reaction: Magnesium and Oxygen
- Balancing the Equation: A Step-by-Step Guide
- Stoichiometry and its Implications
- Practical Applications and Significance
- Beyond the Basic Equation: Considering Reaction Conditions
- Further Exploration: Related Reactions and Compounds
- Conclusion: The Importance of Understanding the Balanced Equation
- Latest Posts
- Latest Posts
- Related Post
The Balanced Chemical Equation for Magnesium Oxide: A Deep Dive
Magnesium oxide, a white hygroscopic solid, finds extensive applications in various industries, from medicine to construction. Understanding its formation, represented by its balanced chemical equation, is crucial for comprehending its properties and applications. This article will delve deep into the balanced chemical equation for magnesium oxide, exploring the reaction mechanism, stoichiometry, and practical implications.
The Reaction: Magnesium and Oxygen
The formation of magnesium oxide (MgO) is a classic example of a combination reaction, also known as a synthesis reaction. In this reaction, two elements, magnesium (Mg) and oxygen (O₂), combine to form a single compound, magnesium oxide. The reaction is highly exothermic, meaning it releases a significant amount of heat and light. This is readily observable when magnesium ribbon is ignited in the presence of air. The bright white light produced is often used in fireworks and flares.
The unbalanced chemical equation representing this reaction is:
Mg + O₂ → MgO
This equation is unbalanced because the number of atoms of each element is not equal on both sides of the arrow. To balance it, we need to adjust the coefficients – the numbers placed before the chemical formulas. Balancing chemical equations is crucial because it ensures that the law of conservation of mass is obeyed. This law states that matter cannot be created or destroyed in a chemical reaction; only rearranged.
Balancing the Equation: A Step-by-Step Guide
To balance the equation, we must ensure that the number of magnesium atoms and oxygen atoms is the same on both the reactant (left-hand side) and product (right-hand side) sides.
-
Start with the most complex molecule: In this case, there isn't a significantly more complex molecule, but we can begin with oxygen.
-
Balance oxygen: There are two oxygen atoms on the reactant side (O₂) and one on the product side (MgO). To balance this, we place a coefficient of 2 in front of MgO:
Mg + O₂ → 2MgO
-
Balance magnesium: Now, we have two magnesium atoms on the product side (2MgO) and only one on the reactant side (Mg). To balance this, we place a coefficient of 2 in front of Mg:
2Mg + O₂ → 2MgO
Now the equation is balanced. We have two magnesium atoms and two oxygen atoms on both the reactant and product sides. This balanced equation accurately represents the stoichiometry of the reaction, showing the precise ratio of reactants and products involved.
Stoichiometry and its Implications
The balanced equation, 2Mg + O₂ → 2MgO, reveals vital stoichiometric information:
-
Reactant Ratio: Two moles of magnesium react with one mole of oxygen gas. This ratio is crucial in determining the amount of each reactant needed for complete reaction and minimizing waste.
-
Product Yield: Two moles of magnesium oxide are produced from this reaction. Knowing the molar mass of magnesium oxide (approximately 40.3 g/mol), we can calculate the theoretical yield of the reaction given specific amounts of reactants.
-
Limiting Reactants: If the amounts of magnesium and oxygen are not in the 2:1 molar ratio, one reactant will be completely consumed before the other. This reactant is called the limiting reactant, as it limits the amount of product formed. The calculation of the limiting reactant is vital for optimizing the reaction.
-
Percent Yield: In real-world scenarios, the actual yield of magnesium oxide may be less than the theoretical yield. This discrepancy is expressed as the percent yield, which provides insight into the efficiency of the reaction. Factors like incomplete reactions or loss during handling can contribute to a lower percent yield.
Practical Applications and Significance
The balanced chemical equation for magnesium oxide's formation is not just a theoretical concept. It has significant practical implications:
-
Industrial Production: Understanding the stoichiometry is essential for the large-scale production of magnesium oxide in industrial settings. Optimizing the reactant ratio ensures efficient use of resources and maximizes product yield.
-
Material Science: Magnesium oxide is a valuable material in various applications. Its high melting point, chemical stability, and electrical insulating properties make it suitable for refractories (materials resistant to high temperatures), electrical insulators, and even in some medical applications. The balanced equation helps predict the amounts of magnesium and oxygen needed to synthesize specific quantities of MgO with desired purity.
-
Environmental Chemistry: Magnesium oxide can be used in environmental remediation, for instance, in neutralizing acidic soils. The balanced equation helps calculate the amount of MgO needed to neutralize a given amount of acid, ensuring effective remediation.
-
Educational Purposes: The reaction between magnesium and oxygen provides a simple and visually striking demonstration of a chemical reaction, making it an excellent teaching tool for illustrating the concepts of balancing chemical equations, stoichiometry, and exothermic reactions in chemistry education.
Beyond the Basic Equation: Considering Reaction Conditions
While the balanced equation provides a fundamental understanding of magnesium oxide formation, the actual reaction is influenced by several factors:
-
Temperature: The reaction between magnesium and oxygen is significantly faster and more vigorous at higher temperatures. At room temperature, the reaction is slow, but the combustion of magnesium ribbon in air demonstrates the rapid reaction at elevated temperatures.
-
Surface Area: A finely divided magnesium powder will react more quickly with oxygen than a solid magnesium ribbon because the increased surface area provides more contact points for the reaction to occur.
-
Presence of Catalysts: While not typically used, certain catalysts could potentially influence the reaction rate.
-
Oxygen Concentration: The reaction rate is also affected by the concentration of oxygen in the air. A pure oxygen environment will result in a much faster and more intense reaction.
Further Exploration: Related Reactions and Compounds
Magnesium oxide's reactivity opens up avenues for further exploration:
-
Reaction with water: Magnesium oxide reacts with water to form magnesium hydroxide, Mg(OH)₂: MgO + H₂O → Mg(OH)₂ This reaction is relatively slow but important in understanding MgO's behavior in moist environments.
-
Reaction with acids: Magnesium oxide is a base and readily reacts with acids to form magnesium salts and water. For example, its reaction with hydrochloric acid (HCl) is: MgO + 2HCl → MgCl₂ + H₂O
-
Formation of other magnesium compounds: Magnesium oxide serves as a precursor for the synthesis of numerous magnesium compounds, highlighting its central role in magnesium chemistry.
Conclusion: The Importance of Understanding the Balanced Equation
The balanced chemical equation for magnesium oxide, 2Mg + O₂ → 2MgO, is more than just a symbolic representation of a reaction. It is a cornerstone for understanding the stoichiometry, predicting reaction yields, and optimizing industrial processes. The reaction's exothermic nature, practical applications, and connections to related chemical reactions underscore its importance in various fields, making its understanding crucial for students and professionals alike. The balanced equation provides a fundamental framework for comprehending the properties and applications of this ubiquitous compound. From fireworks to industrial processes, the seemingly simple equation reveals the intricacies of chemical reactions and the power of stoichiometry in manipulating them.
Latest Posts
Latest Posts
-
The Reaction Has At Least Two Reactants And One Product
Apr 08, 2025
-
What Is Difference Between Impedance And Resistance
Apr 08, 2025
-
What Is The Lcm Of 18 And 45
Apr 08, 2025
-
Difference Between Production And Operation Management
Apr 08, 2025
-
What Is 63 Kg In Lbs
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about Balanced Chemical Equation For Magnesium Oxide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.