Baking Soda An Acid Or Base
Juapaving
Mar 15, 2025 · 5 min read
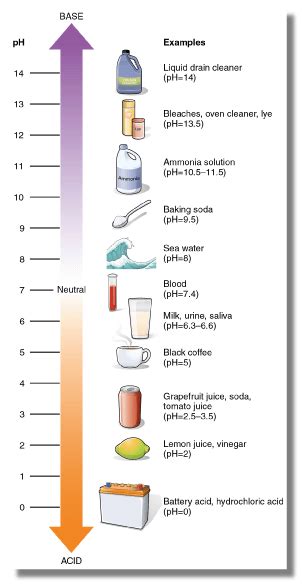
Table of Contents
Baking Soda: An Acid or a Base? Understanding its pH and Reactions
Baking soda, a staple in many kitchens, is a common household item with surprisingly complex chemistry. While many people know it's used in baking and cleaning, fewer understand its fundamental nature: is baking soda an acid or a base? The answer, simply put, is that baking soda is a base. But understanding why requires delving into its chemical properties and reactions. This comprehensive guide will explore baking soda's chemical identity, its interactions with acids, and its various applications, all while emphasizing its fundamental basicity.
The Chemical Identity of Baking Soda
Baking soda, also known as sodium bicarbonate, has the chemical formula NaHCO₃. This formula reveals its ionic nature: it's composed of a sodium cation (Na⁺) and a bicarbonate anion (HCO₃⁻). The bicarbonate ion is the key player in determining baking soda's chemical behavior. It's an amphoteric species, meaning it can act as both an acid and a base, depending on the chemical environment. However, in most common situations, particularly in water, it behaves predominantly as a weak base.
What Makes Baking Soda a Base?
The basicity of baking soda stems from the bicarbonate ion's ability to accept a proton (H⁺) from an acid. This proton acceptance is the defining characteristic of a base according to the Brønsted-Lowry acid-base theory. In water, the bicarbonate ion reacts as follows:
HCO₃⁻ + H₂O ⇌ H₂CO₃ + OH⁻
This equilibrium shows the bicarbonate ion reacting with water to form carbonic acid (H₂CO₃) and a hydroxide ion (OH⁻). The presence of hydroxide ions (OH⁻) is a hallmark of a basic solution. The higher the concentration of OH⁻ ions, the more basic the solution. While this reaction isn't complete, it's sufficient to make baking soda solutions slightly alkaline, resulting in a pH greater than 7.
Baking Soda's Reaction with Acids: The Key to its Baking Power
The remarkable leavening power of baking soda arises from its reaction with acids. This reaction produces carbon dioxide gas (CO₂), which expands, creating the characteristic rise in baked goods. This acid-base reaction is fundamental to understanding baking soda's role in baking.
Understanding the Acid-Base Reaction
When baking soda (NaHCO₃) is combined with an acid, such as vinegar (acetic acid), cream of tartar (potassium bitartrate), or even the acidic components in buttermilk, a neutralization reaction occurs. This reaction produces salt, water, and carbon dioxide gas. A simplified example using acetic acid (CH₃COOH) is shown below:
NaHCO₃ + CH₃COOH → CH₃COONa + H₂O + CO₂
This reaction is crucial in baking because the carbon dioxide gas produced is what causes baked goods to rise. The speed of this reaction depends on several factors including temperature and the strength of the acid used.
The Importance of Acid in Baking with Baking Soda
It's crucial to remember that baking soda requires an acid to produce carbon dioxide and achieve its leavening effect. Using baking soda alone in a recipe won't result in the desired rise. The acid provides the proton (H⁺) that the bicarbonate ion needs to release carbon dioxide. Recipes often include ingredients like lemon juice, buttermilk, or vinegar to provide the necessary acidity. Without these acidic components, the baking soda remains largely unreacted, leading to flat baked goods.
Baking Soda's pH: A Quantitative Measure of Basicity
The pH scale is a logarithmic scale that measures the acidity or basicity of a solution. A pH of 7 is neutral, while values below 7 are acidic and values above 7 are basic (alkaline). A saturated solution of baking soda in water typically has a pH of around 8.3, clearly indicating its basic nature. However, this pH can vary depending on the concentration of the baking soda solution.
Baking Soda vs. Baking Powder: Key Differences
While both baking soda and baking powder are leavening agents, they have crucial differences. Baking soda requires an acid, while baking powder contains both an acid and a base. Baking powder is a complete leavening system, containing sodium bicarbonate (baking soda) and one or more acidic salts. The acid in baking powder reacts with the baking soda when exposed to moisture, producing carbon dioxide. Therefore, baking powder doesn't require the addition of an external acid to work effectively.
Choosing Between Baking Soda and Baking Powder
The choice between baking soda and baking powder depends on the recipe. If a recipe already contains acidic ingredients like buttermilk, vinegar, or lemon juice, baking soda is often preferred for its stronger leavening power. If the recipe lacks sufficient acidity, baking powder is the better choice because it contains its own acid source.
Beyond Baking: Other Applications of Baking Soda's Basicity
Baking soda's basic properties extend beyond its culinary uses. Its ability to neutralize acids makes it valuable in various household and industrial applications:
Cleaning Applications
Baking soda's mild abrasiveness and basicity make it a versatile cleaning agent. It can be used to:
- Deodorize refrigerators and other surfaces: Its basicity helps neutralize acidic odors.
- Clean countertops and sinks: Its mild abrasiveness helps remove stains and grime.
- Clean ovens: Its basicity helps to loosen baked-on food.
- Whiten teeth: While not a substitute for professional cleaning, it can help to remove surface stains.
Other Uses
Beyond cleaning, baking soda's basicity is exploited in:
- Antacids: Its ability to neutralize stomach acid makes it a common ingredient in antacids.
- Fire extinguishers: In some fire extinguishers, baking soda reacts with an acid to produce carbon dioxide, which helps to extinguish the flames.
Safety Precautions
While generally safe, baking soda can pose some risks if mishandled:
- Ingestion in large quantities: Consuming excessive amounts of baking soda can cause electrolyte imbalances and other health problems.
- Inhalation of dust: Inhaling baking soda dust can irritate the lungs and respiratory system.
- Eye contact: Contact with eyes can cause irritation.
Always follow safety guidelines and handle baking soda with care.
Conclusion: Baking Soda – A Versatile Base
Baking soda, with its chemical formula NaHCO₃, is fundamentally a weak base. Its reaction with acids, particularly in baking, is critical for producing carbon dioxide gas, leading to the rise in baked goods. Understanding its basicity helps us appreciate its diverse applications, from baking and cleaning to antacids and fire extinguishers. While safe when used properly, precautions should be taken to avoid ingestion, inhalation, or eye contact. Its versatility, coupled with its readily available nature, makes baking soda a truly remarkable chemical compound.
Latest Posts
Latest Posts
-
Chromosomes Line Up Along The Equator
Mar 15, 2025
-
Is 30 A Prime Number Or Composite
Mar 15, 2025
-
What Are The Factors Of 95
Mar 15, 2025
-
Are Light Waves Transverse Or Longitudinal
Mar 15, 2025
-
What Is The Prime Factorization Of 200
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Baking Soda An Acid Or Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
