Ba Oh 2 Ionic Or Molecular
Juapaving
Mar 10, 2025 · 5 min read
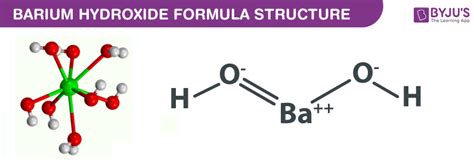
Table of Contents
Ba(OH)₂: Ionic or Molecular? Delving into the Nature of Barium Hydroxide
Determining the nature of a chemical compound as either ionic or molecular is crucial for understanding its properties and behavior. This article will delve deep into the classification of barium hydroxide, Ba(OH)₂, exploring its structure, bonding, and properties to definitively answer whether it's ionic or molecular. We'll also examine related concepts and provide examples to solidify understanding.
Understanding Ionic and Molecular Compounds
Before diving into the specifics of Ba(OH)₂, let's establish a clear understanding of the fundamental differences between ionic and molecular compounds.
Ionic Compounds: These compounds are formed through the electrostatic attraction between oppositely charged ions – cations (positively charged) and anions (negatively charged). This attraction arises from the transfer of electrons from a metal atom to a non-metal atom. Ionic compounds generally exhibit high melting and boiling points, are often crystalline solids at room temperature, and are good conductors of electricity when dissolved in water or molten.
Molecular Compounds: These compounds are formed by the sharing of electrons between atoms, resulting in covalent bonds. This type of bonding typically occurs between non-metal atoms. Molecular compounds usually have lower melting and boiling points compared to ionic compounds and are often liquids or gases at room temperature. They are generally poor conductors of electricity.
The Case of Barium Hydroxide, Ba(OH)₂
Barium hydroxide, Ba(OH)₂, is a white crystalline solid. To classify it, we need to analyze its constituent elements and their bonding behavior.
-
Barium (Ba): Barium is an alkaline earth metal located in Group 2 of the periodic table. Metals, particularly those in Groups 1 and 2, readily lose electrons to achieve a stable electron configuration. Barium readily loses two electrons to form a +2 cation (Ba²⁺).
-
Hydroxide (OH⁻): The hydroxide ion is a polyatomic anion composed of one oxygen atom and one hydrogen atom. The oxygen atom shares electrons with the hydrogen atom, forming a covalent bond within the hydroxide ion. However, the overall hydroxide ion carries a -1 charge due to an imbalance in electron distribution.
The Ionic Bond in Ba(OH)₂: In barium hydroxide, the barium cation (Ba²⁺) and the hydroxide anion (OH⁻) are held together by strong electrostatic forces of attraction. This is the defining characteristic of an ionic bond. The two hydroxide ions are needed to balance the +2 charge of the barium ion, resulting in the formula Ba(OH)₂.
Evidence Supporting the Ionic Nature of Ba(OH)₂
Several observations and properties strongly support the classification of Ba(OH)₂ as an ionic compound:
-
High Melting and Boiling Point: Ba(OH)₂ has a relatively high melting point (around 300-400°C), which is typical of ionic compounds. The strong electrostatic forces between the Ba²⁺ and OH⁻ ions require significant energy to overcome.
-
Crystalline Structure: The crystalline structure of Ba(OH)₂ is a direct consequence of the regular arrangement of ions in the solid state. This ordered arrangement maximizes the electrostatic attractions between oppositely charged ions.
-
Solubility and Conductivity: While less soluble than some other ionic compounds, Ba(OH)₂ dissolves in water to a certain extent. The aqueous solution conducts electricity, indicating the presence of freely moving ions (Ba²⁺ and OH⁻).
-
Electrolysis: When subjected to electrolysis, Ba(OH)₂ undergoes decomposition, yielding barium metal at the cathode and oxygen gas at the anode. This is a characteristic behavior of ionic compounds.
Distinguishing between Ionic and Covalent Character: A Deeper Look
While Ba(OH)₂ is predominantly ionic, it's crucial to acknowledge that the bond within the hydroxide ion (O-H) itself is covalent. The overall compound's nature is determined by the predominant type of bonding. The strong ionic interaction between the Ba²⁺ and OH⁻ ions overshadows the covalent bond within the hydroxide ion.
This concept applies to many compounds. Many compounds exhibit a degree of both ionic and covalent character, demonstrating a spectrum rather than a strict binary classification. The electronegativity difference between the atoms involved plays a crucial role in determining the degree of ionic or covalent character.
Examples of Other Ionic Compounds
Several other compounds exhibit similar ionic bonding characteristics to barium hydroxide. Here are a few examples:
-
Sodium Chloride (NaCl): A classic example of an ionic compound, formed by the electrostatic attraction between sodium cations (Na⁺) and chloride anions (Cl⁻).
-
Magnesium Oxide (MgO): Another example of a highly ionic compound, formed from magnesium cations (Mg²⁺) and oxide anions (O²⁻).
-
Potassium Iodide (KI): A readily soluble ionic compound formed by potassium cations (K⁺) and iodide anions (I⁻).
-
Calcium Carbonate (CaCO₃): Though containing a polyatomic anion (carbonate), the overall bonding is predominantly ionic due to the interaction between calcium cations (Ca²⁺) and carbonate anions (CO₃²⁻).
Applications of Barium Hydroxide
Understanding the properties of Ba(OH)₂ derived from its ionic nature allows us to comprehend its applications:
-
Chemical Synthesis: It serves as a base in various chemical reactions, acting as a reactant or a catalyst.
-
Water Treatment: It's used to adjust the pH of water, especially in industrial processes.
-
Sugar Refining: It participates in the purification of sugar.
-
Laboratory Reagent: Its strong base properties and relatively good solubility make it a useful reagent in many laboratory applications.
Conclusion: Ba(OH)₂ is Predominantly Ionic
In conclusion, based on its structure, bonding, and properties, barium hydroxide, Ba(OH)₂, is predominantly an ionic compound. While it contains a covalent bond within the hydroxide ion, the dominant force holding the compound together is the strong electrostatic attraction between the barium cation (Ba²⁺) and the hydroxide anion (OH⁻). Its high melting point, crystalline structure, conductivity in solution, and behavior during electrolysis all support this classification. This understanding of its ionic nature is key to understanding its chemical reactivity and wide range of applications. Further investigation into the specific crystal structure and spectroscopic data would provide even more detailed insight into the nature of the bonding within this important compound. The knowledge of whether a compound is ionic or molecular fundamentally influences our understanding of its physical and chemical behaviors.
Latest Posts
Latest Posts
-
A Body At Rest Can Have
May 09, 2025
-
The Cell Membrane Is Composed Mainly Of
May 09, 2025
-
What Are The Prime Factorization Of 125
May 09, 2025
-
What Size Is A Mature Follicle
May 09, 2025
-
Is Ribosomes Found In Prokaryotic Or Eukaryotic Cells
May 09, 2025
Related Post
Thank you for visiting our website which covers about Ba Oh 2 Ionic Or Molecular . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.