Atomic Mass Equals The Number Of
Juapaving
Apr 06, 2025 · 5 min read
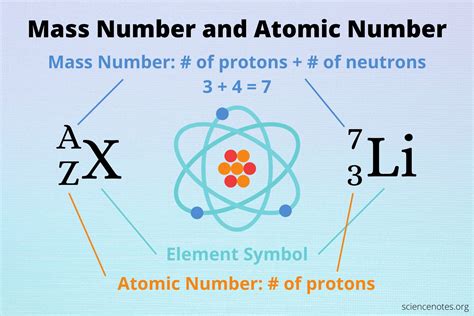
Table of Contents
Atomic Mass: It's Not Just the Number of Protons!
Atomic mass, a fundamental concept in chemistry and physics, is often mistakenly simplified to just the number of protons in an atom. While the number of protons (the atomic number) defines an element, atomic mass is a more nuanced concept encompassing both protons and neutrons. This article delves deep into the intricacies of atomic mass, clarifying its meaning, explaining its calculation, and exploring its significance in various scientific fields. We'll dispel common misconceptions and provide a comprehensive understanding of this critical concept.
Understanding the Components of Atomic Mass
To grasp atomic mass, we need to understand the subatomic particles that constitute an atom:
-
Protons: Positively charged particles found in the atom's nucleus. The number of protons determines the element's identity and is represented by the atomic number (Z).
-
Neutrons: Neutral particles (no charge) also residing in the atom's nucleus. Unlike protons, the number of neutrons can vary within an element, leading to isotopes.
-
Electrons: Negatively charged particles orbiting the nucleus. Their mass is negligible compared to protons and neutrons, so they don't significantly contribute to the atom's overall mass.
The atomic mass (or more accurately, the relative atomic mass) is essentially the weighted average of the masses of all the naturally occurring isotopes of an element. This means it considers both the mass of each isotope and its abundance in nature.
The Role of Isotopes
Isotopes are atoms of the same element (same atomic number) but with different numbers of neutrons. This variation in neutron count results in different mass numbers for each isotope. The mass number (A) is the sum of protons and neutrons in an atom's nucleus.
For example, carbon (atomic number 6) has two main isotopes:
- Carbon-12 (¹²C): 6 protons + 6 neutrons = mass number 12
- Carbon-13 (¹³C): 6 protons + 7 neutrons = mass number 13
Both are carbon atoms because they have six protons. However, they differ in their neutron count and, consequently, their mass.
Calculating Atomic Mass: A Weighted Average
The atomic mass listed on the periodic table isn't the mass of a single atom of a specific isotope. Instead, it's a weighted average reflecting the abundance of each isotope in a naturally occurring sample. The calculation involves:
-
Identifying the isotopes: Determine all the naturally occurring isotopes of the element and their respective mass numbers.
-
Determining isotopic abundances: Find the percentage abundance of each isotope in nature. This data is often obtained through mass spectrometry.
-
Calculating the weighted average: Multiply the mass of each isotope by its abundance (expressed as a decimal), and then sum the results.
Formula:
Atomic mass = (Mass of isotope 1 × Abundance of isotope 1) + (Mass of isotope 2 × Abundance of isotope 2) + ...
Example: Calculating the atomic mass of chlorine
Chlorine has two main isotopes:
- ³⁵Cl (chlorine-35): mass = 34.9689 amu, abundance = 75.77%
- ³⁷Cl (chlorine-37): mass = 36.9659 amu, abundance = 24.23%
Atomic mass of Chlorine = (34.9689 amu × 0.7577) + (36.9659 amu × 0.2423) ≈ 35.45 amu
The value of approximately 35.45 amu is what you'll find on the periodic table for chlorine's atomic mass.
Units of Atomic Mass: amu and g/mol
Atomic mass is typically expressed in atomic mass units (amu). One amu is defined as one-twelfth the mass of a carbon-12 atom. This unit is extremely small, making it impractical for macroscopic measurements.
For larger-scale applications, such as in chemical reactions, the molar mass is used. The molar mass is the mass of one mole (6.022 × 10²³ particles) of an element or compound, expressed in grams per mole (g/mol). The numerical value of the molar mass is the same as the atomic mass, but the units differ.
Significance of Atomic Mass in Various Fields
Atomic mass is not merely an abstract concept; it has profound implications across various scientific disciplines:
-
Chemistry: Atomic mass is crucial for stoichiometric calculations, determining the amounts of reactants and products in chemical reactions. It's fundamental to understanding chemical formulas and balancing chemical equations.
-
Nuclear Physics: Atomic mass plays a critical role in understanding nuclear reactions, such as fission and fusion. The mass defect (the difference between the mass of the nucleus and the sum of the masses of its constituent nucleons) is related to the binding energy holding the nucleus together.
-
Geochemistry and Cosmochemistry: Isotopic ratios and variations in atomic mass provide valuable information about the age of rocks, the origin of meteorites, and the evolution of the Earth and the solar system.
-
Medicine and Biology: Certain isotopes, like ¹⁴C (carbon-14) and ³H (tritium), are used in radioisotope dating and medical imaging techniques. Understanding their atomic mass is crucial for accurate measurements and interpretations.
Common Misconceptions about Atomic Mass
Let's address some prevalent misunderstandings about atomic mass:
-
Atomic mass is not the mass of a single atom: It's a weighted average of the masses of all naturally occurring isotopes.
-
Atomic mass is not simply the number of protons: Neutrons significantly contribute to the atom's mass.
-
Atomic mass is not always a whole number: Due to the weighted averaging of isotopes with different masses, the atomic mass often appears as a decimal.
Conclusion: Atomic Mass – A Deep Dive into the Heart of Matter
Atomic mass is a fundamental concept underpinning our understanding of matter. While often simplified to "the number of protons," a more complete picture reveals its complexity and profound significance. By considering the contributions of both protons and neutrons, and by accounting for the isotopic abundances in nature, we arrive at the weighted average atomic mass that is so crucial in various scientific endeavors. A thorough comprehension of atomic mass is essential for anyone pursuing studies in chemistry, physics, or related fields. This knowledge forms the bedrock for further explorations into the intricacies of the atom and the universe it constitutes. The information provided here helps clarify this essential concept and encourages further exploration into the fascinating world of atomic structure and isotopic variation. Remember to continue your learning and expand your knowledge of this important subject.
Latest Posts
Latest Posts
-
Elements That Are Good Conductors Of Heat And Electricity
Apr 07, 2025
-
Is A Megabyte Larger Than A Gigabyte
Apr 07, 2025
-
Mass Of An Electron In Amu
Apr 07, 2025
-
Simple Diffusion Is Defined As The Movement Of
Apr 07, 2025
-
Why Are Cells Considered The Basic Unit Of Life
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about Atomic Mass Equals The Number Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
