As Temperature Increases The Rate Of Diffusion
Juapaving
Apr 03, 2025 · 6 min read
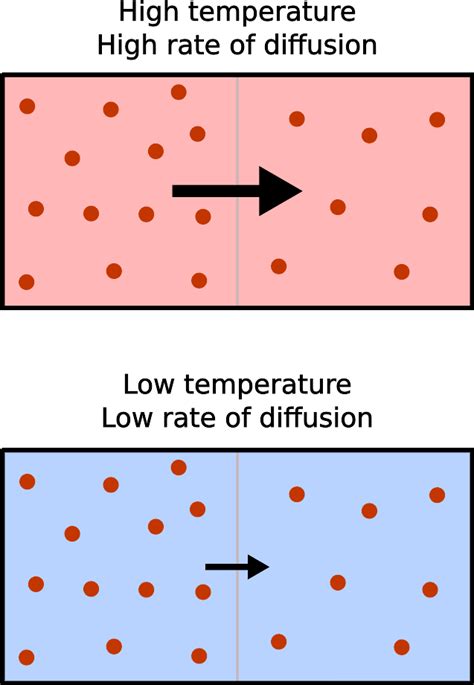
Table of Contents
- As Temperature Increases The Rate Of Diffusion
- Table of Contents
- As Temperature Increases, So Does the Rate of Diffusion: A Deep Dive
- The Kinetic Theory of Matter and its Role in Diffusion
- Higher Temperature, Higher Kinetic Energy
- Increased Particle Collisions and Diffusion
- The Mathematical Relationship: Fick's Law and Temperature
- The Diffusion Coefficient (D) and Temperature Dependence
- Examples of Temperature's Impact on Diffusion in Different Systems
- 1. Gases: The Fastest Diffusers
- 2. Liquids: Moderate Temperature Dependence
- 3. Solids: The Slowest Diffusers
- 4. Biological Systems: Temperature's Critical Role
- 5. Environmental Science: Pollution Dispersion
- Factors Affecting Diffusion Rate Beyond Temperature
- Conclusion: Temperature's Crucial Role in Diffusion
- Latest Posts
- Latest Posts
- Related Post
As Temperature Increases, So Does the Rate of Diffusion: A Deep Dive
Diffusion, the net movement of particles from a region of higher concentration to a region of lower concentration, is a fundamental process in various scientific disciplines, from biology and chemistry to physics and engineering. Understanding the factors that influence diffusion rates is crucial for comprehending a wide array of phenomena, from the transport of oxygen in our blood to the spread of pollutants in the environment. One of the most significant factors affecting the rate of diffusion is temperature. As temperature increases, the rate of diffusion also increases. This relationship is not merely observational; it's deeply rooted in the kinetic theory of matter. This article will explore the intricate connection between temperature and diffusion rate, examining the underlying mechanisms and providing real-world examples.
The Kinetic Theory of Matter and its Role in Diffusion
To fully grasp the temperature-diffusion relationship, we need to understand the kinetic theory of matter. This theory posits that all matter is composed of tiny particles (atoms or molecules) in constant, random motion. The kinetic energy of these particles—their energy of motion—is directly proportional to the absolute temperature (measured in Kelvin). This means that as temperature rises, the particles move faster.
Higher Temperature, Higher Kinetic Energy
Higher temperatures equate to higher average kinetic energy. This is not to say that all particles move at the same speed; there's a distribution of speeds. However, the average speed increases with temperature. This increased speed is the key to understanding the accelerated rate of diffusion.
Increased Particle Collisions and Diffusion
With increased particle speed comes an increase in the frequency and intensity of particle collisions. These collisions are vital to the diffusion process. Particles don't simply travel in straight lines; they constantly bounce off each other and obstacles. These collisions cause the particles to change direction, spreading out more efficiently throughout the available space. Higher kinetic energy leads to more frequent and forceful collisions, accelerating this spreading process and thus, increasing the rate of diffusion.
The Mathematical Relationship: Fick's Law and Temperature
Fick's first law of diffusion provides a quantitative description of the diffusion process. It states that the diffusion flux (the amount of substance diffusing per unit area per unit time) is proportional to the concentration gradient (the change in concentration over distance). While Fick's law doesn't explicitly include temperature, the diffusion coefficient (D) within the law is strongly temperature-dependent.
The Diffusion Coefficient (D) and Temperature Dependence
The diffusion coefficient (D) is a measure of how easily particles can move through a medium. It's influenced by several factors, most notably temperature. The relationship between D and temperature can often be approximated by the Arrhenius equation:
D = D₀ * exp(-Ea/RT)
Where:
- D is the diffusion coefficient
- D₀ is a pre-exponential factor (related to the frequency of particle collisions)
- Ea is the activation energy (the energy barrier particles must overcome to diffuse)
- R is the ideal gas constant
- T is the absolute temperature (in Kelvin)
This equation demonstrates the exponential relationship between temperature and the diffusion coefficient. As temperature (T) increases, the exponential term (-Ea/RT) decreases, leading to a significant increase in the diffusion coefficient (D). This signifies a substantial acceleration in the diffusion rate.
Examples of Temperature's Impact on Diffusion in Different Systems
The effect of temperature on diffusion is evident across a vast range of systems:
1. Gases: The Fastest Diffusers
Gases exhibit the most pronounced temperature dependence on diffusion rates. Gas particles have weak intermolecular forces, allowing them to move relatively freely. A small increase in temperature leads to a significant increase in their kinetic energy and consequently, a dramatic increase in their diffusion rate. Consider the rapid dispersion of a gas leak—the speed of dispersion is markedly faster at higher temperatures.
2. Liquids: Moderate Temperature Dependence
Liquids display a less dramatic, but still significant, increase in diffusion rate with rising temperatures. The liquid particles are closer together than gas particles and experience stronger intermolecular forces. While the increase in kinetic energy with temperature is still substantial, the stronger interactions hinder their movement compared to gases. However, the increase in temperature still allows for more frequent and energetic collisions, thus enhancing diffusion. Think about the dissolving of sugar in water – warmer water dissolves sugar faster than colder water.
3. Solids: The Slowest Diffusers
In solids, the particles are tightly packed, and their movement is significantly restricted. Diffusion in solids is a much slower process compared to gases and liquids. The activation energy (Ea) in the Arrhenius equation is much higher for solids. While an increase in temperature does increase the diffusion rate in solids, the effect is much less pronounced than in gases and liquids. Examples include the diffusion of dopants in semiconductors during manufacturing processes or the very slow diffusion of metals within each other (a phenomenon exploited in techniques like diffusion bonding).
4. Biological Systems: Temperature's Critical Role
Diffusion plays a vital role in biological systems. The transport of oxygen from the lungs to the tissues, the movement of nutrients across cell membranes, and the transmission of nerve impulses are all diffusion-dependent processes. Temperature significantly influences these processes. For example, a fever (elevated body temperature) can speed up metabolic processes, partly because of the increased rate of diffusion of reactants and products within cells. Conversely, hypothermia (low body temperature) can slow down these processes, potentially leading to organ damage.
5. Environmental Science: Pollution Dispersion
The dispersion of pollutants in the environment, whether in air or water, is significantly affected by temperature. Higher temperatures lead to faster diffusion rates, potentially accelerating the spread of pollutants. However, understanding the diffusion process is crucial for developing effective pollution control strategies. Predictive modeling of pollutant dispersion often incorporates temperature as a critical parameter.
Factors Affecting Diffusion Rate Beyond Temperature
While temperature is a primary factor influencing diffusion, it's not the only one. Several other factors also play a significant role:
-
Concentration Gradient: A steeper concentration gradient results in a faster diffusion rate. The larger the difference in concentration between two regions, the greater the driving force for diffusion.
-
Medium's Properties: The nature of the medium through which diffusion occurs significantly influences the diffusion rate. For example, diffusion is faster in less viscous media (like water) compared to more viscous media (like honey). Porosity and tortuosity of the medium also affect the diffusion pathway.
-
Particle Size and Shape: Larger particles diffuse more slowly than smaller particles. The shape of the particle also plays a role; spherical particles typically diffuse faster than irregularly shaped particles.
-
Pressure: In gases, increased pressure leads to increased collision frequency, potentially enhancing the diffusion rate. The effect is more complex in liquids and solids.
Conclusion: Temperature's Crucial Role in Diffusion
The relationship between temperature and the rate of diffusion is a fundamental concept with far-reaching implications. The increased kinetic energy of particles at higher temperatures leads to more frequent and energetic collisions, accelerating the process of diffusion across various systems. This relationship is well-described by the Arrhenius equation and has practical applications in diverse fields, from biological systems to environmental science and materials engineering. Understanding this relationship is key to comprehending many natural processes and developing innovative technologies. Further research continues to refine our understanding of the intricacies of diffusion and its dependence on temperature and other factors, paving the way for advancements in diverse scientific disciplines.
Latest Posts
Latest Posts
-
What Is The Smallest Complete Unit Of An Element
Apr 10, 2025
-
Which Of The Following Is Soluble In Water
Apr 10, 2025
-
Where Is The Buffer Region Of A Titration Curve
Apr 10, 2025
-
What Bacteria Live In The Root Nodules Of Legumes
Apr 10, 2025
-
How Many Amps For 220 Volts
Apr 10, 2025
Related Post
Thank you for visiting our website which covers about As Temperature Increases The Rate Of Diffusion . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.