Are Polar Bonds Stronger Than Nonpolar
Juapaving
Apr 01, 2025 · 5 min read
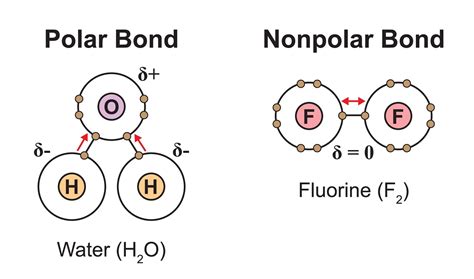
Table of Contents
Are Polar Bonds Stronger Than Nonpolar Bonds? A Deep Dive into Chemical Bonding
The question of whether polar bonds are stronger than nonpolar bonds doesn't have a simple yes or no answer. The strength of a chemical bond is a complex issue influenced by several factors, and while polarity plays a role, it's not the sole determinant. This in-depth exploration will dissect the nature of polar and nonpolar bonds, explore the forces at play, and ultimately provide a nuanced understanding of their relative strengths.
Understanding Bond Polarity: The Role of Electronegativity
The key to understanding the difference lies in the concept of electronegativity. Electronegativity is a measure of an atom's ability to attract shared electrons in a chemical bond. Elements with high electronegativity, like oxygen and fluorine, strongly attract electrons. Elements with low electronegativity, like alkali metals and alkaline earth metals, have a weaker pull on electrons.
-
Nonpolar Covalent Bonds: In a nonpolar covalent bond, two atoms share electrons equally. This typically occurs when two atoms of the same element bond (e.g., H₂ or O₂), or when atoms with very similar electronegativities bond (e.g., C-H bonds). The electron cloud is evenly distributed between the atoms.
-
Polar Covalent Bonds: In a polar covalent bond, the electrons are shared unequally. This happens when atoms with significantly different electronegativities bond. The atom with higher electronegativity attracts the shared electrons more strongly, creating a partial negative charge (δ-) on that atom and a partial positive charge (δ+) on the other atom. This results in a dipole moment – a separation of charge within the molecule. Examples include the O-H bond in water (H₂O) and the C-O bond in carbon dioxide (CO₂).
Factors Affecting Bond Strength: Beyond Polarity
While polarity influences bond strength, several other factors significantly impact it:
1. Bond Length: The Distance Matters
Bond length is the average distance between the nuclei of two bonded atoms. Shorter bonds are generally stronger. This is because the closer the atoms are, the stronger the electrostatic attraction between their nuclei and the shared electrons. However, the relationship isn't strictly linear; other factors influence the optimal bond length.
2. Bond Order: Multiple Bonds are Stronger
Bond order refers to the number of chemical bonds between two atoms. A single bond has a bond order of 1, a double bond has a bond order of 2, and a triple bond has a bond order of 3. Multiple bonds are shorter and stronger than single bonds. The increased electron density between the atoms leads to a stronger attractive force. For instance, a triple bond (like in N₂) is significantly stronger than a single bond (like in Cl₂).
3. Atomic Size: Larger Atoms, Weaker Bonds
The size of the atoms involved also plays a critical role. Larger atoms have their valence electrons farther from the nucleus, leading to weaker electrostatic attraction. Consequently, bonds between larger atoms are generally weaker than those between smaller atoms.
4. Hybridization: Orbital Overlap Influences Strength
The hybridization of atomic orbitals involved in bond formation also affects bond strength. Different hybridization schemes (sp, sp², sp³, etc.) lead to different degrees of orbital overlap, which in turn influences bond strength and length. For instance, sp hybridized orbitals lead to stronger and shorter bonds compared to sp³ hybridized orbitals.
5. Resonance: Electron Delocalization Strengthens Bonds
In molecules with resonance structures, electrons are delocalized across multiple bonds. This delocalization strengthens the overall bonding, as the electrons are spread out over a larger region, resulting in a lower overall energy state for the molecule. Benzene (C₆H₆) is a classic example of a molecule with resonance structures leading to enhanced bond strength.
Comparing Strengths: A Nuanced Perspective
It's inaccurate to categorically state that all polar bonds are stronger or weaker than all nonpolar bonds. The relative strengths depend on the interplay of all the factors mentioned above.
-
A Nonpolar Bond Can Be Stronger: Consider a triple bond between two carbon atoms (C≡C) versus a single polar bond between a carbon and oxygen atom (C-O). The triple bond, despite being nonpolar, is significantly stronger due to its higher bond order and shorter bond length.
-
A Polar Bond Can Be Stronger: Conversely, a polar bond between oxygen and hydrogen (O-H) in water is relatively strong due to the high electronegativity difference, leading to strong dipole-dipole interactions that enhance the overall bond stability.
-
Polarity's Contribution: The polarity of a bond does contribute to its overall strength by introducing additional intermolecular forces. These forces, such as dipole-dipole interactions and hydrogen bonding (a specific type of dipole-dipole interaction), significantly impact the physical properties (like boiling point and melting point) of the substance but do not directly affect the intrinsic strength of the covalent bond itself.
Conclusion: Context is Crucial
The strength of a chemical bond is a multifaceted issue governed by several factors, and polarity is just one piece of the puzzle. While polarity can contribute to overall bond strength by influencing intermolecular interactions, it's essential to consider bond length, bond order, atomic size, hybridization, and resonance effects. A direct comparison between polar and nonpolar bonds requires a case-by-case analysis considering all these factors. It's not a simple matter of one being universally stronger than the other. The context of the specific chemical bonds in question is crucial in determining their relative strengths. Therefore, you cannot make a definitive statement about the general strength comparison between these types of bonds. Instead, a detailed examination of each specific bond is necessary.
Latest Posts
Latest Posts
-
How Long Is 40 Cm In Inches
Apr 02, 2025
-
5 Letter Words Starting With R A
Apr 02, 2025
-
How Many Hours Are In 210 Minutes
Apr 02, 2025
-
Does A Gas Have A Definite Volume
Apr 02, 2025
-
Which Base Is Found Only In Rna
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Are Polar Bonds Stronger Than Nonpolar . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
