An Atom's Mass Number Equals The Number Of
Juapaving
May 09, 2025 · 6 min read
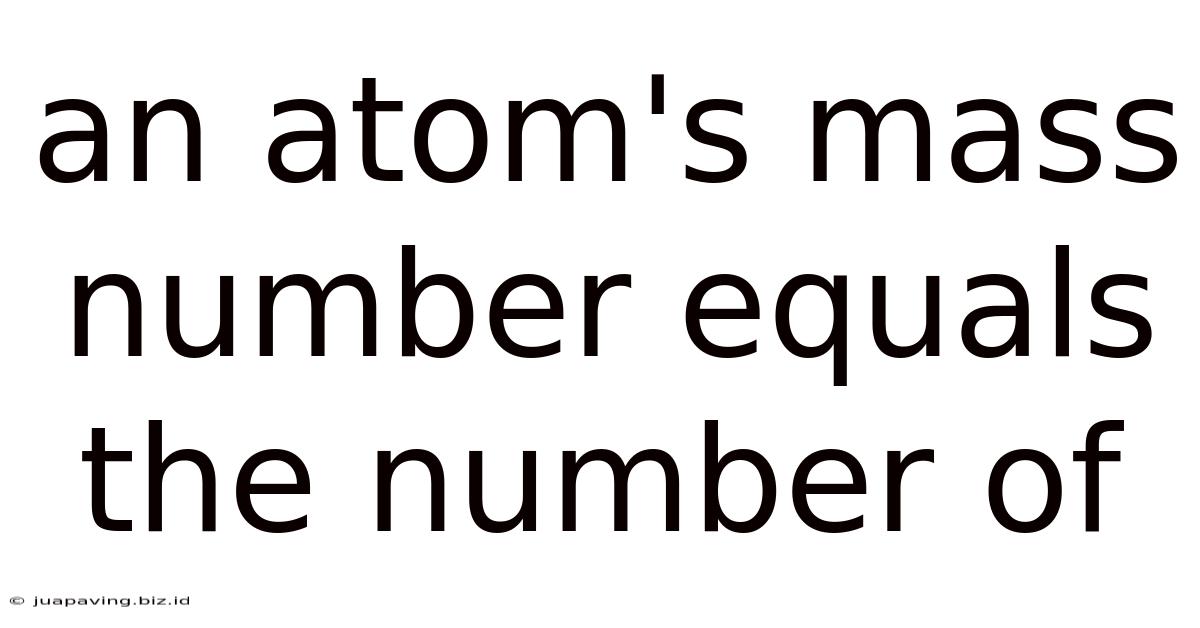
Table of Contents
An Atom's Mass Number Equals the Number of Protons and Neutrons
Atoms, the fundamental building blocks of matter, are incredibly complex entities. Understanding their structure is key to comprehending the properties of all substances, from the air we breathe to the stars in the sky. One crucial aspect of atomic structure is the mass number, a value that tells us much about the atom's identity and behavior. This article will delve into the core concept: an atom's mass number equals the number of protons and neutrons. We'll explore the roles of protons, neutrons, and electrons, explaining how they contribute to an atom's overall mass and properties, and examining the implications of isotopic variations.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before diving into the mass number, let's solidify our understanding of the subatomic particles that constitute an atom. These are:
Protons: The Positive Charge Carriers
Protons reside within the atom's nucleus, the dense central region. They possess a positive electrical charge and contribute significantly to the atom's overall mass. Crucially, the number of protons defines the atomic number, a unique identifier for each element. For instance, all hydrogen atoms have one proton (atomic number 1), all helium atoms have two (atomic number 2), and so on. The atomic number is fundamental because it determines the element's chemical properties and its position on the periodic table.
Neutrons: The Neutral Mass Contributors
Also located in the atom's nucleus, neutrons are particles with no electrical charge. Their primary contribution is to the atom's mass. Unlike protons, the number of neutrons can vary within an element, leading to the existence of isotopes (discussed in detail below). The neutrons play a crucial role in stabilizing the nucleus, preventing the electrostatic repulsion between positively charged protons from causing the nucleus to disintegrate.
Electrons: The Negatively Charged Orbitals
Electrons are far lighter than protons and neutrons, orbiting the nucleus in shells or energy levels. They carry a negative electrical charge, balancing the positive charge of the protons in a neutral atom. The arrangement of electrons in these shells determines the atom's chemical reactivity and how it interacts with other atoms to form molecules and compounds. The number of electrons in the outermost shell, the valence electrons, is particularly important in determining an atom's bonding behavior. While electrons contribute minimally to an atom's mass, their role in chemical reactions is paramount.
Mass Number: The Sum of Protons and Neutrons
Now, let's get to the heart of the matter: the mass number. The mass number (A) of an atom is simply the total number of protons and neutrons in its nucleus. It's represented as a superscript to the left of the element's symbol in isotopic notation (e.g., ¹²C for carbon-12). The equation summarizing this is:
A = Z + N
Where:
- A is the mass number
- Z is the atomic number (number of protons)
- N is the number of neutrons
This equation highlights the direct relationship between the mass number and the number of protons and neutrons. The mass number provides a convenient way to characterize the different isotopes of an element.
Isotopes: Variations in Neutron Number
Isotopes are atoms of the same element that have the same number of protons (atomic number) but differ in the number of neutrons. This means they have the same atomic number (Z) but different mass numbers (A). For example, carbon has three naturally occurring isotopes:
- ¹²C (Carbon-12): 6 protons + 6 neutrons = Mass number 12
- ¹³C (Carbon-13): 6 protons + 7 neutrons = Mass number 13
- ¹⁴C (Carbon-14): 6 protons + 8 neutrons = Mass number 14
All three are carbon atoms because they all have 6 protons, but they differ in their neutron count and therefore their mass numbers. The properties of isotopes are largely similar chemically because they have the same number of electrons, but their physical properties, such as mass and radioactivity (in the case of some isotopes), can vary significantly. Carbon-14, for instance, is radioactive and is used in radiocarbon dating.
Atomic Mass and Mass Number: A Subtle Distinction
It's essential to differentiate between mass number and atomic mass. The mass number is a whole number representing the sum of protons and neutrons in a specific isotope. The atomic mass (or atomic weight), on the other hand, is the weighted average of the masses of all naturally occurring isotopes of an element. It's not a whole number because it considers the relative abundance of each isotope. For example, the atomic mass of carbon is approximately 12.011 amu (atomic mass units), reflecting the contribution of ¹²C, ¹³C, and ¹⁴C isotopes and their relative abundances.
Applications of Mass Number and Isotopes
Understanding the mass number and the concept of isotopes has widespread applications across various scientific fields:
Nuclear Chemistry and Physics:
- Nuclear reactions: Mass numbers are crucial in predicting the products and understanding the energy changes involved in nuclear reactions such as fission and fusion.
- Radioactive dating: Isotopes like carbon-14, uranium-238, and potassium-40 are used extensively in radiometric dating to determine the age of ancient artifacts, rocks, and fossils.
- Nuclear medicine: Radioactive isotopes are used in medical imaging and therapy, such as PET scans and radiation treatments for cancer.
Chemistry:
- Mass spectrometry: This technique allows scientists to determine the mass-to-charge ratio of ions, enabling the identification and quantification of different isotopes in a sample. This is vital in analytical chemistry and environmental monitoring.
- Isotope effects: The slight differences in mass between isotopes can affect the rates of chemical reactions, a phenomenon known as the isotope effect. This has applications in various fields, including biochemistry and environmental science.
Other Fields
The principles relating to mass number and isotopic variations also extend to fields such as geology, archaeology, and environmental science, offering insights into past climates, geological processes, and pollution sources.
Conclusion: A Fundamental Concept in Atomic Structure
The statement "an atom's mass number equals the number of protons and neutrons" is a cornerstone of our understanding of atomic structure. This seemingly simple concept opens doors to a deep understanding of the elements, their isotopes, and their diverse applications in science and technology. By grasping the interplay between protons, neutrons, and electrons, and their contributions to the mass number, we gain invaluable insights into the fundamental building blocks of the universe and their role in shaping our world. The concept is further amplified by considering the implications of isotopic variation, illustrating the diversity within elements and the power of scientific tools to analyze these variations. The applications are far-reaching, proving the foundational importance of understanding the atom's mass number and its relationship to the subatomic particles.
Latest Posts
Latest Posts
-
What Is 1 8 Of 64
May 09, 2025
-
What Is The Function Of Root Hairs
May 09, 2025
-
Greatest Common Factor Of 27 And 63
May 09, 2025
-
Cellulose Starch And Glycogen Are Examples Of
May 09, 2025
-
The End Product Of The Calvin Cycle Is
May 09, 2025
Related Post
Thank you for visiting our website which covers about An Atom's Mass Number Equals The Number Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.