All Of The Following Nucleotide Bases Are Pyrimidines Except
Juapaving
Mar 22, 2025 · 6 min read
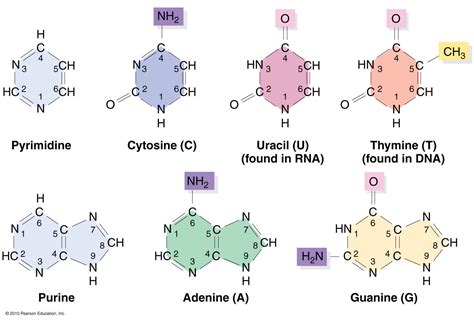
Table of Contents
All of the Following Nucleotide Bases are Pyrimidines Except… Adenine! Understanding Nucleotide Structure and Function
Understanding the fundamental building blocks of DNA and RNA is crucial for comprehending the intricacies of life itself. These molecules, the blueprints of life, are constructed from nucleotides, which are composed of a sugar molecule, a phosphate group, and a nitrogenous base. The nitrogenous bases are further categorized into two groups: purines and pyrimidines. This article will delve into the structural differences between these bases, focusing specifically on the question: All of the following nucleotide bases are pyrimidines except… The answer, as you'll soon discover, is adenine.
Understanding Purines and Pyrimidines: The Foundation of Nucleic Acids
The nitrogenous bases are the information-carrying components of DNA and RNA. They pair up to form the characteristic double helix structure of DNA and the single-stranded structure of RNA. These bases are categorized into two distinct groups based on their chemical structure:
-
Purines: These bases possess a double-ring structure, consisting of a six-membered ring fused to a five-membered ring. The two most common purines found in DNA and RNA are adenine (A) and guanine (G).
-
Pyrimidines: These bases have a single six-membered ring structure. The pyrimidines found in DNA and RNA are cytosine (C), thymine (T), and uracil (U). It's important to note that thymine is primarily found in DNA, while uracil replaces thymine in RNA.
The Chemical Structure: A Closer Look
The difference in the ring structure directly influences the base-pairing capabilities of the nucleotides. Let's examine the chemical structures:
Adenine (A) - The Purine Exception
Adenine, with its characteristic double-ring structure (a six-membered ring fused to a five-membered ring), is a purine. It differs significantly from the pyrimidines in its structural complexity. This structural difference is crucial for its ability to pair specifically with thymine (in DNA) or uracil (in RNA) through hydrogen bonding.
Cytosine (C) - A Pyrimidine Base
Cytosine, a pyrimidine, possesses a single six-membered ring. Its structure allows it to form three hydrogen bonds with guanine, contributing to the stability of the DNA double helix. Its presence in both DNA and RNA underscores its importance in genetic information storage and transfer.
Guanine (G) - Another Purine Base
Like adenine, guanine is a purine with a double-ring structure. It forms three hydrogen bonds with cytosine, playing a vital role in the stability and structural integrity of DNA and RNA molecules. Its role in base pairing is fundamental to the accurate replication and transcription of genetic material.
Thymine (T) - A DNA-Specific Pyrimidine
Thymine, a pyrimidine found exclusively in DNA, differs from uracil by the presence of a methyl group. This subtle chemical difference is significant in terms of its recognition and binding with adenine. Its pairing with adenine through two hydrogen bonds is essential for maintaining the integrity of the DNA molecule.
Uracil (U) - An RNA-Specific Pyrimidine
Uracil, a pyrimidine found only in RNA, is structurally similar to thymine, but lacks the methyl group. This difference is often cited as a possible factor contributing to the higher susceptibility of RNA to degradation compared to DNA. Its pairing with adenine is crucial for RNA structure and function.
Base Pairing: The Key to Genetic Information
The specific base pairing between purines and pyrimidines is fundamental to the function of DNA and RNA. This pairing, driven by hydrogen bonding, follows strict rules:
-
Adenine (A) pairs with Thymine (T) in DNA or Uracil (U) in RNA (A-T or A-U). These pairings involve two hydrogen bonds.
-
Guanine (G) pairs with Cytosine (C) (G-C). These pairings involve three hydrogen bonds, making them slightly stronger than A-T or A-U pairings.
This complementary base pairing allows for the accurate replication of DNA and the transcription of DNA into RNA, ensuring the faithful transmission of genetic information.
The Significance of the Distinction: Why it Matters
The distinction between purines and pyrimidines is not merely a matter of chemical structure. It has profound implications for:
-
DNA Replication: Accurate replication of DNA requires the precise pairing of purines and pyrimidines. Any errors in this process can lead to mutations with potentially harmful consequences.
-
RNA Transcription: The synthesis of RNA from a DNA template depends on the accurate base pairing between the DNA and RNA nucleotides. Errors in transcription can lead to the production of non-functional proteins.
-
Protein Synthesis: The genetic information encoded in DNA and RNA ultimately directs the synthesis of proteins. The sequence of bases determines the sequence of amino acids, which in turn dictates the protein's structure and function. Any errors in the nucleotide sequence can lead to the production of malfunctioning proteins.
-
Drug Design: The unique structural features of purines and pyrimidines are exploited in the design of many drugs that target specific aspects of nucleic acid metabolism. For example, many antiviral and anticancer drugs interfere with the synthesis or function of nucleic acids.
Beyond the Basics: Exploring Advanced Concepts
The fundamental concepts discussed above provide a solid foundation for understanding nucleic acids. However, further exploration can lead to a deeper appreciation of the complexities involved. This includes:
-
Modifications of Nucleotide Bases: Nucleotide bases can undergo various modifications, such as methylation, which can affect gene expression and DNA stability.
-
Non-canonical Base Pairs: While the standard base pairings (A-T/U, G-C) are predominant, non-canonical base pairs can also form under certain conditions.
-
The Role of Nucleotide Analogs: Synthetic analogs of nucleotide bases are used in various research and medical applications, including gene therapy and cancer treatment. These analogs can mimic the structure of natural bases, enabling researchers to study the mechanisms of DNA replication, repair, and gene expression.
-
Nucleic Acid Secondary and Tertiary Structures: The secondary and tertiary structures of DNA and RNA are influenced by the interactions between the nucleotide bases, leading to complex three-dimensional conformations crucial for their functionality. Understanding these structures helps in understanding how nucleic acids interact with proteins and other molecules.
Conclusion: Adenine – The Key Exception and its Significance
To reiterate the main point: All of the following nucleotide bases are pyrimidines except adenine. Adenine's unique purine structure, differing significantly from the single-ring pyrimidine structure, is critical to its specific pairing with thymine (in DNA) and uracil (in RNA). This difference in chemical structure underlies the fundamental mechanisms of DNA replication, RNA transcription, and protein synthesis. Understanding the distinction between purines and pyrimidines, and the specific roles of each base, is fundamental to a complete understanding of molecular biology and the very foundation of life itself. The seemingly simple question of which base is not a pyrimidine opens a door to a vast and fascinating world of molecular intricacies, highlighting the exquisite precision and complexity of life's blueprint.
Latest Posts
Latest Posts
-
The Price Elasticity Of Demand Is A Negative Number Because
May 09, 2025
-
Lock And Key Model Vs Induced Fit Model
May 09, 2025
-
What Element Has An Atomic Number Of 13
May 09, 2025
-
What Is A Global Variable In C
May 09, 2025
-
What Organelles Are Only In Animal Cells
May 09, 2025
Related Post
Thank you for visiting our website which covers about All Of The Following Nucleotide Bases Are Pyrimidines Except . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.