Alkenes Can Be Converted To Alcohols By Hydroboration Oxidation
Juapaving
Mar 22, 2025 · 6 min read
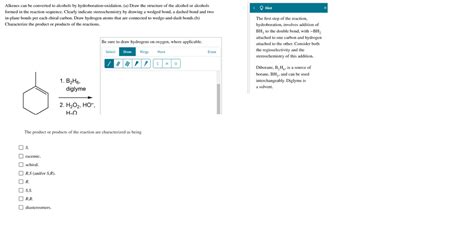
Table of Contents
Alkenes to Alcohols: A Comprehensive Guide to Hydroboration-Oxidation
Alkenes, unsaturated hydrocarbons characterized by a carbon-carbon double bond, are versatile building blocks in organic chemistry. Their reactivity allows for a wide array of transformations, one of the most significant being their conversion to alcohols. This conversion can be achieved through various methods, but the hydroboration-oxidation reaction stands out for its regio- and stereoselectivity, making it a powerful and widely used technique. This comprehensive guide delves into the intricacies of this reaction, exploring its mechanism, regioselectivity, stereochemistry, and applications.
Understanding the Hydroboration-Oxidation Reaction
The hydroboration-oxidation reaction is a two-step process that transforms an alkene into an alcohol. It's a highly regioselective and stereospecific reaction, meaning the position and spatial arrangement of the hydroxyl group (-OH) in the product are predictable and controlled.
Step 1: Hydroboration
This step involves the addition of borane (BH₃) or a borane derivative, such as 9-borabicyclo[3.3.1]nonane (9-BBN) or disiamylborane, across the alkene's double bond. Borane acts as an electrophile, while the alkene acts as a nucleophile. The reaction proceeds via a concerted mechanism, meaning the boron and hydrogen atoms add to the double bond simultaneously. This concerted addition is crucial in determining the regio- and stereochemistry of the product.
Key Features of Hydroboration:
- Regioselectivity: Borane adds to the less substituted carbon atom of the double bond (anti-Markovnikov addition). This is in contrast to the Markovnikov addition observed in other alkene reactions, such as acid-catalyzed hydration. This anti-Markovnikov addition is a hallmark of hydroboration.
- Stereochemistry: The addition of borane is syn, meaning the boron and hydrogen atoms add to the same side of the double bond. This syn addition results in a cis addition to the alkene.
- Mechanism: The reaction proceeds through a four-centered transition state, where the boron atom and one hydrogen atom simultaneously bond to the carbons of the double bond.
Step 2: Oxidation
The organoborane intermediate formed in the hydroboration step is then oxidized using an oxidizing agent, typically hydrogen peroxide (H₂O₂) in the presence of a base like sodium hydroxide (NaOH). This oxidation step replaces the boron atom with a hydroxyl group (-OH), resulting in the formation of an alcohol.
Key Features of Oxidation:
- Mechanism: The oxidation proceeds via a series of steps involving the formation of a peroxide intermediate followed by its rearrangement and hydrolysis to yield the alcohol. The base is crucial for this process, facilitating the nucleophilic attack on the boron atom.
- Stereochemistry: The oxidation step preserves the stereochemistry established in the hydroboration step. Therefore, if the hydroboration yielded a cis addition product, the final alcohol will also have a cis configuration.
Regioselectivity in Hydroboration-Oxidation: The Anti-Markovnikov Rule
The most striking characteristic of hydroboration-oxidation is its anti-Markovnikov regioselectivity. Unlike many other alkene addition reactions that follow Markovnikov's rule (the addition of a proton to the carbon atom with more hydrogen atoms), hydroboration adds the boron atom (and subsequently the hydroxyl group) to the less substituted carbon atom. This is explained by the steric hindrance around the more substituted carbon atom, making it less accessible to the bulky borane molecule. The transition state leading to the anti-Markovnikov product is less sterically crowded and thus energetically favored.
Examples illustrating Anti-Markovnikov Addition:
Let's consider the hydroboration-oxidation of propene:
-
Hydroboration: BH₃ adds to the less substituted carbon (the terminal carbon) of the propene double bond, forming an organoborane intermediate.
-
Oxidation: Oxidation with H₂O₂/NaOH replaces the boron atom with a hydroxyl group, resulting in the formation of 1-propanol, not 2-propanol as would be predicted by Markovnikov's rule.
Another example is the hydroboration-oxidation of 2-methyl-2-butene. This reaction would lead to the formation of 3-methyl-2-butanol. The hydroxyl group ends up on the less substituted carbon atom, demonstrating again the anti-Markovnikov regioselectivity of the reaction.
Stereochemistry in Hydroboration-Oxidation: Syn Addition
The hydroboration step is a syn addition, meaning that the boron and hydrogen atoms add to the same face of the alkene double bond. This syn addition is a direct consequence of the concerted mechanism. The transition state involves a cyclic four-membered structure, forcing the boron and hydrogen to approach from the same side. This syn addition is preserved throughout the oxidation step, leading to the formation of a cis alcohol in the case of cyclic alkenes or a specific stereocenter in the case of other alkenes.
Examples Illustrating Syn Addition:
Consider the hydroboration-oxidation of cis-2-butene:
The reaction yields (2R,3R)-butane-2,3-diol and (2S,3S)-butane-2,3-diol as a mixture of enantiomers. The syn addition of borane leads to the formation of the same stereocenter at both positions.
Similarly, if we start with trans-2-butene, the reaction will give a mixture of meso-2,3-butanediol. Again, the syn addition of borane is preserved during the entire reaction.
Practical Applications of Hydroboration-Oxidation
The high regio- and stereoselectivity of the hydroboration-oxidation reaction makes it an invaluable tool in organic synthesis. It is widely used in the preparation of a wide variety of alcohols, many of which are important intermediates in the synthesis of more complex molecules.
-
Synthesis of Alcohols: It is particularly useful for the synthesis of primary alcohols from terminal alkenes.
-
Synthesis of Chiral Alcohols: It can be utilized in asymmetric synthesis, generating chiral alcohols with high enantiomeric excess (ee) when chiral borane derivatives are used.
-
Preparation of Pharmaceutical Intermediates: Many pharmaceuticals and natural products contain alcohol functional groups, and hydroboration-oxidation is a key step in their synthesis.
-
Industrial Applications: The reaction is robust enough for large-scale synthesis and finds applications in industrial settings for the production of various chemicals and materials.
Choosing the Right Borane Reagent
The choice of borane reagent can affect the reaction's outcome, particularly its rate and selectivity. Different borane reagents exhibit varying steric properties and reactivities.
-
BH₃ (Borane): The simplest borane reagent but can be difficult to handle due to its pyrophoric nature. Often, BH₃ is complexed with a solvent, such as THF.
-
9-BBN (9-Borabicyclo[3.3.1]nonane): A sterically hindered borane reagent, which shows higher selectivity for less hindered alkenes.
-
Disiamylborane: Another sterically hindered reagent, which often provides higher selectivity and is less reactive than BH₃.
The choice of the borane reagent depends on the specific alkene and the desired outcome of the reaction. The steric bulk and reactivity profile of the borane reagent have to be considered.
Limitations of Hydroboration-Oxidation
Despite its advantages, hydroboration-oxidation does have some limitations:
-
Sensitivity to Air and Moisture: Boranes are highly reactive towards air and moisture, requiring anhydrous conditions for the reaction.
-
Not Suitable for Highly Substituted Alkenes: Steric hindrance can affect the reaction efficiency with heavily substituted alkenes.
-
Potential for Side Reactions: The oxidation step might lead to side reactions under certain conditions. Careful control of the reaction conditions is essential to minimize these side reactions.
Conclusion
Hydroboration-oxidation is a powerful and versatile method for converting alkenes into alcohols. Its high regio- and stereoselectivity, along with its relatively mild reaction conditions, makes it a preferred method in many organic synthesis applications. Understanding the reaction mechanism, regioselectivity, stereochemistry, and practical considerations ensures successful implementation of this important transformation in organic chemistry. Furthermore, careful consideration of the appropriate borane reagent is crucial for optimizing reaction yield and selectivity. While some limitations exist, the benefits of hydroboration-oxidation far outweigh the drawbacks, solidifying its place as a fundamental technique in organic synthesis laboratories worldwide.
Latest Posts
Latest Posts
-
4 6 Rounded To The Nearest Tenth
May 09, 2025
-
If X Is A Multiple Of 18 And 60
May 09, 2025
-
Does Light Require A Medium To Travel
May 09, 2025
-
Cuanto Es 15 Inches En Centimetros
May 09, 2025
-
The Least Common Multiple Of 12 And 8
May 09, 2025
Related Post
Thank you for visiting our website which covers about Alkenes Can Be Converted To Alcohols By Hydroboration Oxidation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.