A Substance That Forms Hydroxide Ions In A Solution
Juapaving
Mar 12, 2025 · 5 min read
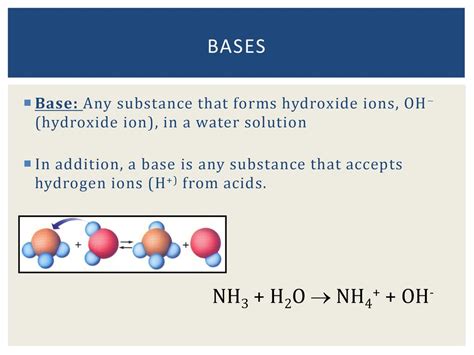
Table of Contents
A Substance That Forms Hydroxide Ions in a Solution: A Deep Dive into Bases
A substance that forms hydroxide ions (OH⁻) in a solution is known as a base. Understanding bases is crucial in chemistry, impacting various fields from industrial processes to biological systems. This comprehensive article will explore the characteristics, types, reactions, and applications of bases, providing a thorough understanding of their significance in the world around us.
Defining Bases: More Than Just Hydroxide Ions
While the classic definition centers around hydroxide ion formation, the concept of a base has evolved. The Arrhenius definition, which focuses solely on hydroxide ion production in aqueous solutions, is limited. A broader and more encompassing definition comes from Brønsted-Lowry theory, which defines a base as a proton acceptor. This means a base accepts a hydrogen ion (H⁺) from an acid. This broader definition allows for the classification of substances as bases even in non-aqueous solutions. The Lewis theory further expands this, defining a base as an electron pair donor. This theory is the most general and encompasses all other definitions.
Key Characteristics of Bases
- Taste: Bases generally taste bitter, although this should never be tested due to the potential dangers involved.
- Feel: Many bases feel slippery or soapy to the touch, a result of their reaction with skin oils. Again, direct contact should be avoided due to potential harm.
- pH: Bases have a pH greater than 7. The pH scale measures the concentration of hydrogen ions in a solution; a higher pH indicates a lower concentration of hydrogen ions and a higher concentration of hydroxide ions.
- Reaction with Acids: Bases react with acids in a process called neutralization, producing water and a salt. This reaction is fundamental to acid-base chemistry.
- Electrical Conductivity: Aqueous solutions of strong bases are good conductors of electricity due to the presence of freely moving ions.
Types of Bases
Bases are categorized based on their strength and solubility:
Strong Bases
Strong bases completely dissociate in water, meaning all of the base molecules break apart into ions. Common examples include:
-
Group 1 Hydroxides (Alkali Metal Hydroxides): These include sodium hydroxide (NaOH), potassium hydroxide (KOH), lithium hydroxide (LiOH), etc. These are highly soluble and readily release hydroxide ions in solution. They are extremely corrosive and require careful handling.
-
Group 2 Hydroxides (Alkaline Earth Metal Hydroxides): These include calcium hydroxide (Ca(OH)₂), barium hydroxide (Ba(OH)₂), etc. While less soluble than Group 1 hydroxides, they still produce significant concentrations of hydroxide ions when dissolved.
Weak Bases
Weak bases only partially dissociate in water. This means only a fraction of the base molecules break down into ions, resulting in a lower concentration of hydroxide ions compared to strong bases. Examples include:
-
Ammonia (NH₃): Ammonia reacts with water to form ammonium ions (NH₄⁺) and hydroxide ions (OH⁻), but the equilibrium lies far to the left, meaning most of the ammonia remains undissociated.
-
Many Organic Amines: Organic amines, containing nitrogen atoms bonded to alkyl groups, also act as weak bases. Their basicity depends on the structure of the molecule.
Other Base Types
Beyond the strong and weak classifications, other categories exist:
-
Superbases: These are exceptionally strong bases, often exceeding the basicity of common strong bases. They are used in specialized chemical reactions.
-
Lewis Bases: As mentioned earlier, Lewis bases are electron pair donors, capable of forming coordinate covalent bonds with Lewis acids (electron pair acceptors). This definition expands the concept of basicity beyond hydroxide ion formation.
Reactions Involving Bases
Bases participate in a variety of crucial reactions:
Neutralization Reactions
The most well-known reaction of bases is their neutralization reaction with acids. This reaction produces water and a salt:
Acid + Base → Salt + Water
For example:
HCl (aq) + NaOH (aq) → NaCl (aq) + H₂O (l)
This reaction is essential for titrations, a quantitative method to determine the concentration of an unknown acid or base.
Saponification
Bases, particularly strong bases like sodium hydroxide, react with fats and oils in a process called saponification. This reaction produces soap and glycerol. This process has been used for centuries in soap making.
Reaction with Metals
Some bases, particularly molten or concentrated solutions, react with certain metals, producing hydrogen gas.
Applications of Bases
Bases have numerous applications in various industries and aspects of daily life:
Industrial Applications
-
Chemical Synthesis: Bases are crucial reagents in countless chemical syntheses, often acting as catalysts or reactants.
-
Manufacturing: Bases are used in the production of various materials, including soaps, detergents, paper, and textiles.
-
Water Treatment: Bases are used to adjust the pH of water, making it suitable for drinking or industrial use.
Everyday Applications
-
Cleaning Products: Many household cleaning products contain bases, which help to dissolve grease and other substances.
-
Food Industry: Bases are used in food processing, often as pH regulators or to neutralize acidic compounds.
-
Medicine: Certain bases are used in medications, and many biological systems rely on pH balance, which is regulated by base-acid equilibria.
Safety Precautions
It is crucial to remember that many bases are corrosive and can cause severe burns. Always handle bases with appropriate safety measures:
- Wear protective equipment: This includes gloves, goggles, and lab coats.
- Work in a well-ventilated area: Some base reactions produce harmful fumes.
- Neutralize spills carefully: Use appropriate neutralizing agents and follow safety protocols.
- Store bases properly: Keep them in sealed containers, away from incompatible materials.
Conclusion: The Ubiquitous Role of Bases
Bases, defined broadly as proton acceptors or electron pair donors, play a vital role in chemistry and numerous applications across various fields. Understanding their properties, reactions, and safety precautions is crucial for anyone working with these essential chemical substances. From industrial processes to everyday life, the importance of bases cannot be overstated. Their impact on our world is vast and continues to expand as scientific understanding and technological advancements progress. This in-depth exploration serves as a valuable resource for anyone seeking to deepen their knowledge of this fundamental area of chemistry. Further research into specific types of bases and their individual applications will provide even greater insight into their significance.
Latest Posts
Latest Posts
-
How Is The Circulatory System Similar To A Road And Highway System
May 09, 2025
-
Least Common Multiple Of 42 And 24
May 09, 2025
-
The Male Accessory Glands Include The
May 09, 2025
-
The Starting Molecule For Glycolysis Is
May 09, 2025
-
How Many Months In Three Years
May 09, 2025
Related Post
Thank you for visiting our website which covers about A Substance That Forms Hydroxide Ions In A Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.