8 Protons 8 Neutrons 8 Electrons
Juapaving
Mar 18, 2025 · 6 min read
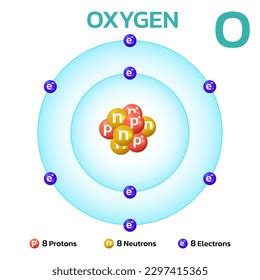
Table of Contents
8 Protons, 8 Neutrons, 8 Electrons: Delving into the World of Oxygen
Oxygen. The very word conjures images of life, breath, and the vibrant hues of a sunset. But beyond its essential role in our survival and the beauty it adorns our world with, lies a fascinating subatomic story: a tale of eight protons, eight neutrons, and eight electrons. This seemingly simple arrangement defines the most common isotope of oxygen, and understanding its structure unlocks a deeper comprehension of its properties and the crucial role it plays in the universe.
The Atomic Structure: A Closer Look
At the heart of every oxygen atom lies its nucleus, a dense core containing protons and neutrons. These particles, collectively known as nucleons, are bound together by the strong nuclear force, an incredibly powerful interaction that overcomes the electrostatic repulsion between the positively charged protons. In the most abundant isotope of oxygen, Oxygen-16 (¹⁶O), we find eight protons and eight neutrons. This combination gives oxygen its atomic number, 8 – the number of protons uniquely identifies it as oxygen on the periodic table.
Surrounding this nucleus is a cloud of electrons, negatively charged particles that are significantly lighter than protons and neutrons. These electrons occupy specific energy levels or shells, orbiting the nucleus at varying distances. In a neutral oxygen atom, there are eight electrons, balancing the positive charge of the eight protons. This balance of positive and negative charges is what makes the atom electrically neutral.
The Significance of Eight
The number eight isn't arbitrary. It's a consequence of the fundamental principles governing atomic structure and the filling of electron shells. The first electron shell can hold a maximum of two electrons, while the second shell can accommodate up to eight. Oxygen, with its eight electrons, has its first shell completely filled with two electrons and its second shell partially filled with six. This incomplete outer shell is crucial to understanding oxygen's chemical reactivity.
Oxygen's Chemical Behavior: A Reactive Element
Oxygen's incomplete outer electron shell makes it highly reactive. Atoms strive for stability, often achieved by having a full outer electron shell. Oxygen can achieve this stability by either gaining two electrons or sharing electrons with other atoms. This tendency to form chemical bonds is what drives oxygen's participation in a vast array of chemical reactions.
Oxidation and Reduction
Oxygen's reactivity is prominently displayed in oxidation, a process where oxygen atoms gain electrons and other atoms lose them. This electron transfer is a fundamental aspect of many chemical reactions, including combustion and respiration. For example, the rusting of iron involves oxygen reacting with iron, forming iron oxide. The opposite process is reduction, where an atom gains electrons, often occurring simultaneously with oxidation (a redox reaction).
Formation of Chemical Bonds
Oxygen's ability to form chemical bonds is crucial to its role in life. It readily forms covalent bonds, sharing electrons with other atoms to achieve a stable electron configuration. In water (H₂O), oxygen shares electrons with two hydrogen atoms, forming a strong, stable molecule. This bond is crucial for life, as water is the essential solvent for biological processes. Oxygen also forms covalent bonds with carbon atoms in organic molecules, enabling the existence of complex life forms.
Isotopes of Oxygen: Variations on a Theme
While ¹⁶O (8 protons, 8 neutrons) is the most common isotope of oxygen, other isotopes exist, differing in the number of neutrons. These isotopes are still oxygen because they have the same number of protons, but they have different mass numbers (the sum of protons and neutrons). For example, ¹⁷O has nine neutrons and ¹⁸O has ten neutrons. These isotopes have similar chemical properties but slightly different physical properties, such as mass and density. The differences in neutron number can affect their stability, with some isotopes being radioactive and decaying over time.
Significance of Isotopes in Scientific Research
The different isotopes of oxygen have found applications in various scientific fields, including archaeology, paleoclimatology, and geochemistry. The ratios of different oxygen isotopes in materials can provide insights into past climates, water sources, and geological processes. By analyzing these isotope ratios, scientists can reconstruct past environments and understand how they have changed over time.
Oxygen's Role in Life and the Environment
Oxygen's importance to life is undeniable. It's a vital component of respiration, the process by which organisms convert glucose into energy, releasing carbon dioxide and water as byproducts. This process sustains life by providing the energy needed for biological functions. Oxygen's role extends far beyond respiration, forming integral parts of countless biological molecules, such as proteins and lipids.
The Oxygen Cycle: A Dynamic Equilibrium
Oxygen is not a static element; it's constantly cycling through the atmosphere, hydrosphere, and biosphere. Photosynthesis, carried out by plants and other photosynthetic organisms, is the primary source of atmospheric oxygen. During photosynthesis, sunlight's energy is used to convert carbon dioxide and water into glucose and oxygen. This oxygen is then released into the atmosphere, fueling the respiration of other organisms. This continuous interplay between photosynthesis and respiration maintains the delicate balance of oxygen in our environment.
Environmental Impact of Oxygen
While oxygen is essential for life, changes in its concentration can have significant environmental consequences. Increased atmospheric oxygen can lead to the formation of ozone in the stratosphere, which helps shield the Earth from harmful ultraviolet radiation. However, ground-level ozone is a pollutant, contributing to respiratory problems. Conversely, decreases in oxygen concentration, such as those occurring in polluted waters, can lead to the death of aquatic organisms.
Oxygen in Industry and Technology
Beyond its biological significance, oxygen plays a vital role in various industrial processes and technologies. It's used in welding and cutting, where it supports combustion, producing high temperatures needed to melt metals. It's also used in the steel industry to remove impurities from iron, enhancing the steel's strength and durability. In healthcare, oxygen is administered to patients with respiratory difficulties, aiding breathing and increasing oxygen levels in the blood.
Oxygen in Space Exploration
Even in space exploration, oxygen plays a critical role. It's essential for the survival of astronauts, providing the oxygen they need for respiration. It's also used as an oxidizer in rocket propulsion systems, where it combines with fuel to generate thrust.
Conclusion: An Element of Life
Eight protons, eight neutrons, eight electrons – this simple atomic configuration defines an element that is fundamental to life on Earth and crucial to numerous technological advancements. Understanding the structure and properties of oxygen, its chemical behavior, and its roles in various processes gives us a deeper appreciation for its importance and the intricate web of life it sustains. From the air we breathe to the processes that sustain life, oxygen remains a captivating and indispensable element, a testament to the elegance and power of fundamental scientific principles. Further research into oxygen's behavior and interactions will continue to reveal its multifaceted nature and enhance our understanding of the universe around us. The simple elegance of eight protons, eight neutrons, and eight electrons forms a story far richer and more complex than one might initially imagine.
Latest Posts
Latest Posts
-
Names Of Shapes With 5 Sides
Mar 18, 2025
-
Steam Produces More Severe Burns Than Boiling Water
Mar 18, 2025
-
Is The Number 3 Prime Or Composite
Mar 18, 2025
-
The Type Of Waves That Require Medium To Pass
Mar 18, 2025
-
What Are The Factors Of 79
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about 8 Protons 8 Neutrons 8 Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
