1 Universal Mass Unit Eual To
Juapaving
Mar 04, 2025 · 5 min read
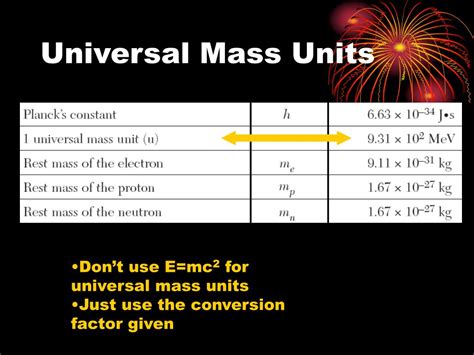
Table of Contents
1 Universal Mass Unit: Exploring the Unified Atomic Mass Unit (amu)
The concept of a universal mass unit is crucial in various scientific fields, especially in chemistry and physics. While there isn't a single, universally recognized unit called a "universal mass unit," the unified atomic mass unit (amu), also known as the dalton (Da), serves as the closest practical equivalent. This article delves deep into the amu, explaining its definition, significance, applications, and historical context. We'll explore its role in understanding atomic and molecular masses, its connection to Avogadro's number, and its implications for various scientific calculations.
Defining the Unified Atomic Mass Unit (amu)
The unified atomic mass unit (amu) or dalton (Da) is defined as one-twelfth the mass of a carbon-12 atom (¹²C). This specific isotope of carbon was chosen as the standard due to its abundance and relatively stable nuclear structure. It provides a consistent and readily reproducible benchmark for measuring the mass of atoms and molecules.
It's important to understand that the amu isn't a directly measurable unit like a kilogram or a gram. Instead, it's a relative unit, based on the mass ratio between atoms. Scientists have determined the mass of a ¹²C atom through sophisticated techniques, allowing for the precise calculation of the amu. The currently accepted value is approximately 1.66053906660 × 10⁻²⁷ kg.
Why Carbon-12?
The selection of carbon-12 as the standard wasn't arbitrary. Several factors contributed to its choice:
- Abundance: Carbon-12 is a relatively abundant isotope of carbon, making it easily accessible for experimentation.
- Stability: Its nuclear structure is exceptionally stable, ensuring consistent mass measurements.
- Availability: Carbon-12 is readily available in pure form, facilitating accurate measurements.
- Technological advancements: Advances in mass spectrometry allowed for precise measurements of the mass of ¹²C, providing a solid foundation for defining the amu.
Significance of the amu in Chemistry and Physics
The amu plays a pivotal role in numerous scientific calculations and concepts:
-
Atomic Mass: The atomic mass of an element is expressed in amu. It represents the average mass of all isotopes of that element, weighted by their natural abundance. For example, the atomic mass of chlorine is approximately 35.45 amu because it exists as a mixture of chlorine-35 and chlorine-37 isotopes.
-
Molecular Mass: The molecular mass of a compound is the sum of the atomic masses of all the atoms in the molecule, expressed in amu. This calculation is fundamental to stoichiometry and chemical reactions.
-
Molar Mass: The molar mass of a substance is the mass of one mole of that substance, expressed in grams per mole (g/mol). It's numerically equivalent to the molecular mass expressed in amu. For instance, the molar mass of water (H₂O) is approximately 18 g/mol, reflecting its molecular mass of approximately 18 amu. This equivalence stems from Avogadro's number.
-
Avogadro's Number and the Mole: Avogadro's number (approximately 6.022 × 10²³) is defined as the number of atoms in 12 grams of carbon-12. This number forms the foundation of the mole concept, a crucial unit in chemistry for relating macroscopic quantities of substances to their microscopic constituents. The amu's link to carbon-12 is intrinsically connected to Avogadro's number and the mole.
Applications of the amu
The unified atomic mass unit finds applications across a wide spectrum of scientific disciplines:
-
Mass Spectrometry: Mass spectrometry is a powerful analytical technique used to determine the mass-to-charge ratio of ions. The results are often expressed in amu, providing invaluable insights into the composition of molecules and their isotopic ratios.
-
Nuclear Physics: In nuclear physics, amu is essential for calculating nuclear binding energies, masses of isotopes, and understanding nuclear reactions.
-
Biochemistry: In biochemistry, amu is vital for determining the molecular masses of proteins, peptides, and other biomolecules. This information is essential for understanding protein structure and function.
-
Materials Science: The amu plays a significant role in characterizing materials at the atomic level, aiding in the design and development of novel materials with specific properties.
Historical Context and Evolution of the amu
The concept of a relative atomic mass predates the current definition of the amu. Early chemists used various standards, including hydrogen and oxygen, to establish relative atomic weights. However, these standards lacked precision and consistency.
The shift towards carbon-12 as the standard was a significant improvement. The International Union of Pure and Applied Chemistry (IUPAC) officially adopted the unified atomic mass unit in 1961, replacing earlier, less precise definitions. This adoption brought about standardization and enhanced accuracy in chemical and physical measurements.
Comparing amu to other Mass Units
While the amu is a fundamental unit in many scientific contexts, it's important to understand its relationship to other mass units:
-
Kilogram (kg): The kilogram is the SI base unit of mass. The amu is a much smaller unit, related to the kilogram by the conversion factor mentioned earlier (1 amu ≈ 1.66053906660 × 10⁻²⁷ kg).
-
Gram (g): The gram is a commonly used unit of mass. The conversion between amu and gram involves Avogadro's number. One mole of a substance with a molecular mass of x amu has a mass of x grams.
-
Electronvolt (eV): The electronvolt is an energy unit, but it can be related to mass through Einstein's famous equation, E=mc². The conversion between amu and eV involves fundamental constants like the speed of light and the electron charge.
The Future of the amu
The unified atomic mass unit continues to be a cornerstone of chemical and physical measurements. As technology advances, the precision of the amu's determination will likely improve further, leading to even more accurate measurements in various scientific domains. The continued reliance on carbon-12 as the standard ensures consistency and facilitates interoperability across different research groups and laboratories globally.
Conclusion
The unified atomic mass unit (amu) or dalton (Da) serves as a crucial standard for measuring atomic and molecular masses. Its definition, based on one-twelfth the mass of a carbon-12 atom, provides a precise and reproducible benchmark essential for numerous scientific calculations and applications. From mass spectrometry to biochemistry, the amu's role in accurately determining the mass of atoms and molecules remains indispensable in advancing our understanding of the physical and chemical world. Its connection to Avogadro's number and the mole concept further solidifies its importance in chemistry and related fields. As scientific understanding and technology continue to evolve, the amu will undoubtedly remain a fundamental unit for precise measurements, fostering further discoveries and advancements.
Latest Posts
Latest Posts
-
How Many Hours In 7 Days
Mar 04, 2025
-
Example Of Electrical Energy Converted Into Chemical Energy
Mar 04, 2025
-
How Many Centimeters Is 6 Ft
Mar 04, 2025
-
Which Of The Following Statements Is Not True
Mar 04, 2025
-
What Is 2 3 As A Percent
Mar 04, 2025
Related Post
Thank you for visiting our website which covers about 1 Universal Mass Unit Eual To . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
